As per Intent Market Research, the Zopiclone Market was valued at USD 1.6 billion in 2023 and will surpass USD 2.7 billion by 2030; growing at a CAGR of 8.3% during 2024 - 2030.
The Zopiclone market is a significant segment of the global pharmaceutical industry, primarily driven by the rising prevalence of insomnia and other sleep disorders. Zopiclone, a non-benzodiazepine hypnotic medication, is widely prescribed for short-term treatment of insomnia and other sleep-related issues. It works by enhancing the activity of neurotransmitters in the brain to promote relaxation and sleep. The market for Zopiclone is expanding, owing to the increasing awareness about sleep disorders and the growing aging population, who are more prone to conditions such as insomnia. Furthermore, the demand for Zopiclone is influenced by the ongoing development of generics and the introduction of various formulations that cater to different patient needs.
The market can be divided into different segments, such as generic and branded Zopiclone, each serving a distinct set of consumers based on factors such as cost, brand preference, and availability. Generic Zopiclone has seen substantial growth due to its affordability, while branded versions still dominate the market in some regions where consumers prioritize brand trust and established efficacy. Formulations such as tablets and oral solutions further cater to diverse patient needs, as some patients prefer tablets for their convenience, while others may opt for oral solutions due to difficulty swallowing tablets. These various factors collectively drive the growth and diversification of the Zopiclone market.
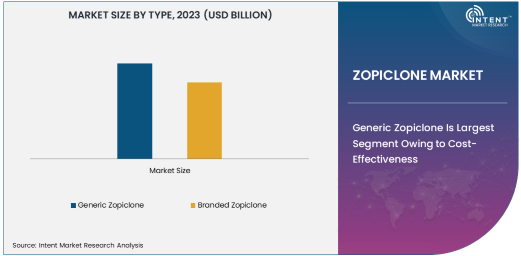
Generic Zopiclone Is Largest Segment Owing to Cost-Effectiveness
Generic Zopiclone is the largest subsegment of the Zopiclone market, primarily due to its affordability and wide accessibility. The availability of generic formulations has made Zopiclone accessible to a larger segment of the population, especially in emerging markets where the cost of branded drugs can be prohibitive. Generic versions of Zopiclone are typically priced lower than their branded counterparts, making them an attractive option for consumers who are seeking cost-effective solutions for their sleep disorders. With the increasing adoption of generics across the pharmaceutical industry, particularly in markets such as North America and Europe, the demand for generic Zopiclone continues to rise, driving growth in this subsegment.
Moreover, regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved multiple generic versions of Zopiclone, which has further contributed to the segment’s growth. The presence of well-established pharmaceutical companies manufacturing generic Zopiclone ensures the availability of high-quality products at competitive prices, thereby boosting their market penetration. As the global preference for generic medications continues to rise, driven by the increasing cost sensitivity of healthcare systems, generic Zopiclone is expected to maintain its dominant position in the market.
Tablets Segment Is Largest Due to Convenience and Ease of Use
The tablet form of Zopiclone is the largest subsegment in terms of formulation type, owing to its convenience, ease of use, and wide acceptance among patients. Tablets are a preferred option for patients who find it easier to take a solid form of medication compared to liquids or syrups. The convenience of swallowing a tablet at bedtime without the need for additional preparation or measuring has contributed to the widespread use of Zopiclone tablets in the treatment of insomnia. The tablet formulation is also commonly prescribed by healthcare professionals due to its precise dosing, which ensures better control over medication administration.
Additionally, the tablet form of Zopiclone is typically more stable and has a longer shelf life than liquid formulations, which makes it an attractive option for pharmaceutical companies and healthcare providers. The prevalence of tablets in the Zopiclone market is further supported by the growing global demand for self-administered, easy-to-take medications, especially for conditions like insomnia, which are often treated on an outpatient basis. As such, the tablet formulation is likely to continue leading the market, meeting the needs of both patients and healthcare providers.
Hospitals Are Largest End-Use Industry Due to High Prescription Volume
Hospitals are the largest end-use industry in the Zopiclone market, largely due to the high volume of prescriptions issued to patients with sleep disorders and related conditions. Hospitals, particularly those with specialized sleep disorder units, are key players in prescribing medications like Zopiclone for short-term insomnia management. Patients in hospitals often require quick and effective treatment for sleep disturbances, which is why Zopiclone, with its fast-acting sedative properties, is frequently prescribed in these settings. Furthermore, hospitals have the resources to administer and monitor Zopiclone treatment for patients with more complex health issues, ensuring that patients receive the appropriate care for their condition.
In addition to being used for patients in sleep disorder units, Zopiclone is also prescribed in hospital settings for individuals recovering from surgery or undergoing other treatments that may interfere with normal sleep patterns. The comprehensive care provided in hospitals, alongside the high number of patients receiving sleep-related treatments, solidifies the hospital sector as the dominant end-user for Zopiclone products. As healthcare institutions continue to prioritize effective treatment for sleep disturbances, hospitals will remain the primary source of demand for Zopiclone.
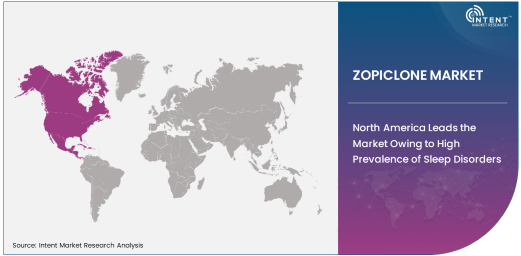
North America Leads the Market Owing to High Prevalence of Sleep Disorders
North America holds the largest market share for Zopiclone, driven by the high prevalence of sleep disorders, particularly insomnia, and the growing awareness about the importance of sleep health. The region has a large population of individuals suffering from sleep disturbances, many of whom seek pharmaceutical interventions like Zopiclone to manage their conditions. Furthermore, the established healthcare infrastructure, access to medications, and strong healthcare policies that prioritize the treatment of sleep disorders have contributed to North America’s dominance in the market. In addition, the presence of leading pharmaceutical companies and the widespread availability of both generic and branded Zopiclone formulations have reinforced the region's market leadership.
In North America, healthcare providers are increasingly recognizing the importance of addressing sleep-related issues, and as a result, the demand for medications like Zopiclone is expected to rise. This growth is further supported by the rising geriatric population in the region, who are more prone to insomnia and other sleep-related disorders. As the demand for effective treatments for sleep disorders increases, North America is expected to continue leading the Zopiclone market, setting trends for the rest of the world.
Leading Companies and Competitive Landscape
The Zopiclone market is highly competitive, with several prominent players focusing on the development and distribution of both generic and branded formulations. Leading companies in this market include Sun Pharma, Mylan N.V., Hikma Pharmaceuticals, and Apotex Inc., among others. These companies are continuously expanding their portfolios by offering both branded and generic versions of Zopiclone to cater to the growing demand across various regions. The competitive landscape is characterized by a focus on affordability, quality, and availability, particularly in emerging markets where cost-sensitive patients increasingly prefer generic medications.
Additionally, pharmaceutical companies are increasingly focusing on enhancing the bioavailability and effectiveness of their Zopiclone formulations to attract healthcare providers and patients alike. Strategic mergers, partnerships, and acquisitions are also common in the market as companies seek to expand their market presence and improve their product offerings. As the demand for sleep disorder treatments grows globally, these companies are likely to continue competing on pricing, accessibility, and product innovation, making the Zopiclone market an evolving and dynamic segment within the global pharmaceutical industry.
Recent Developments:
- Hikma Pharmaceuticals launched a new generic version of Zopiclone tablets, providing more affordable options for the treatment of insomnia in North America.
- Teva Pharmaceuticals received regulatory approval for its Zopiclone oral solution formulation, offering an alternative for patients with difficulty swallowing tablets.
- Mylan N.V. (Viatris) expanded its Zopiclone portfolio with the launch of a lower-dose tablet aimed at treating mild cases of insomnia.
- Sun Pharmaceutical Industries Ltd. entered into a strategic partnership with healthcare providers to offer Zopiclone as part of their sleep disorder management programs.
- Dr. Reddy's Laboratories announced the release of its generic Zopiclone tablets in Europe, aiming to address the growing demand for affordable insomnia treatments.
List of Leading Companies:
- Hikma Pharmaceuticals
- Mylan N.V. (now part of Viatris)
- Teva Pharmaceuticals
- Sandoz (Novartis)
- Sun Pharmaceutical Industries Ltd.
- Lupin Pharmaceuticals
- Aurobindo Pharma
- Pfizer Inc.
- Dr. Reddy's Laboratories
- Torrent Pharmaceuticals
- Zydus Cadila
- Glenmark Pharmaceuticals
- Apotex Inc.
- Cipla Limited
- Emcure Pharmaceuticals
Report Scope:
|
Report Features |
Description |
|
Market Size (2023) |
USD 1.6 Billion |
|
Forecasted Value (2030) |
USD 2.7 Billion |
|
CAGR (2024 – 2030) |
8.3% |
|
Base Year for Estimation |
2023 |
|
Historic Year |
2022 |
|
Forecast Period |
2024 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Zopiclone Market by Type (Generic Zopiclone, Branded Zopiclone), by Formulation (Tablets, Oral Solution), and by End-Use Industry (Hospitals, Clinics, Homecare) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Aeroqual Ltd., Alphasense, Amphenol Corporation, City Technology Ltd., Dynament Ltd., Figaro Engineering, GFG Instrumentation, Honeywell International Inc., K-Flow Instruments, Mettler Toledo, RKI Instruments, Inc., SenseAir and SGX Sensortech |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Zopiclone Market, by Type (Market Size & Forecast: USD Million, 2022 – 2030) |
|
4.1. Generic Zopiclone |
|
4.2. Branded Zopiclone |
|
5. Zopiclone Market, by Formulation (Market Size & Forecast: USD Million, 2022 – 2030) |
|
5.1. Tablets |
|
5.2. Oral Solution |
|
6. Zopiclone Market, by End-Use Industry (Market Size & Forecast: USD Million, 2022 – 2030) |
|
6.1. Hospitals |
|
6.2. Clinics |
|
6.3. Homecare |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2022 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Zopiclone Market, by Type |
|
7.2.7. North America Zopiclone Market, by Formulation |
|
7.2.8. North America Zopiclone Market, by End-Use Industry |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Zopiclone Market, by Type |
|
7.2.9.1.2. US Zopiclone Market, by Formulation |
|
7.2.9.1.3. US Zopiclone Market, by End-Use Industry |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. Boston Scientific |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. Medtronic |
|
9.3. Abbott Laboratories |
|
9.4. Cook Medical |
|
9.5. Terumo Corporation |
|
9.6. Cardiovascular Systems, Inc. |
|
9.7. Cardiatis |
|
9.8. B. Braun Melsungen AG |
|
9.9. Stryker Corporation |
|
9.10. Biotronik |
|
9.11. Acandis GmbH & Co. KG |
|
9.12. Merit Medical Systems |
|
9.13. Endosmart GmbH |
|
9.14. APT Medical |
|
9.15. Volcano Corporation (now part of Philips) |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Zopiclone Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Zopiclone Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Zopiclone Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
