As per Intent Market Research, the Ventricular Assist Devices Market was valued at USD 1.2 billion in 2024-e and will surpass USD 2.5 billion by 2030; growing at a CAGR of 13.3% during 2025 - 2030.
Ventricular Assist Devices (VADs) are mechanical pumps used to assist heart function in patients suffering from severe heart failure. These devices are particularly critical for patients waiting for a heart transplant or for those who are not candidates for heart transplants. VADs work by helping the heart pump blood, alleviating the symptoms of heart failure and enhancing the quality of life for patients. As heart disease continues to be a leading cause of mortality globally, the demand for VADs is expected to rise, especially as the prevalence of heart failure increases with an aging population.
The VAD market has seen significant technological advancements over the years, leading to improvements in device performance, durability, and patient comfort. Newer VAD systems are smaller, more efficient, and can be implanted with less invasive procedures, making them more accessible to a broader range of patients. The market's growth is also fueled by the increasing number of heart failure cases, which are driving the adoption of VADs across hospitals, specialized heart clinics, and homecare settings.
Left Ventricular Assist Devices (LVADs) Lead the Market Owing to Widespread Use in Heart Failure Treatment
Left Ventricular Assist Devices (LVADs) dominate the ventricular assist devices market, accounting for the largest share of the market. LVADs are used primarily for patients with left-sided heart failure, which is the most common form of heart failure. These devices are designed to assist the left ventricle in pumping blood to the rest of the body, relieving the symptoms of heart failure and providing a bridge to heart transplantation or, in some cases, long-term support for patients who are not candidates for a transplant.
The high prevalence of left-sided heart failure and the proven efficacy of LVADs in improving patients’ survival rates and quality of life contribute significantly to their dominance in the market. As the understanding of heart failure continues to advance, LVADs are expected to remain the most commonly used type of ventricular assist device in clinical practice.
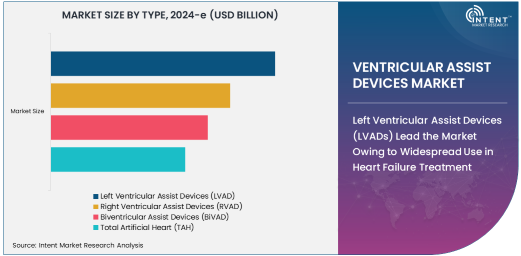
Biventricular Assist Devices (BiVADs) Expanding in Complex Heart Failure Cases
Biventricular Assist Devices (BiVADs) are seeing increased adoption for treating patients with severe biventricular heart failure. BiVADs provide support to both the left and right ventricles, making them ideal for patients with advanced heart failure affecting both sides of the heart. These devices are often used when other treatment options have been exhausted, providing crucial support for patients who are not candidates for heart transplantation or are waiting for a transplant.
While the BiVAD segment remains smaller compared to LVADs and RVADs, it is expanding as medical advancements allow for more complex heart failure cases to be treated effectively. The increasing recognition of BiVADs' potential to improve survival rates and quality of life in these critically ill patients is driving growth in this segment.
Pulsatile Flow Devices Lead the Flow Type Segment Due to Proven Efficacy in Long-Term Use
Pulsatile flow devices are leading the flow type segment, owing to their proven efficacy and long-term success in supporting heart function. These devices mimic the natural pumping action of the heart, providing pulsatile blood flow that is more similar to the body’s natural circulation. Pulsatile flow VADs have been the standard in clinical practice for many years, offering a reliable and well-established treatment option for patients with advanced heart failure.
Despite the rise of non-pulsatile flow devices, pulsatile flow devices remain the preferred choice in many clinical settings due to their well-documented clinical outcomes and ability to provide better circulation in certain patients. The sustained success and reliability of pulsatile flow devices ensure their continued dominance in the market.
Hospitals Remain the Largest End-User Segment Due to High Demand for Critical Care
Hospitals continue to be the largest end-user of ventricular assist devices due to their critical role in treating severe heart failure patients. Hospitals, especially those with specialized cardiology departments, use VADs extensively in intensive care units (ICUs) and other specialized heart failure treatment units. The need for VADs in hospitals is driven by the high incidence of heart failure cases, the availability of advanced medical infrastructure, and the ability to monitor patients closely during VAD implantation and operation.
As the demand for heart failure treatments continues to rise, hospitals will remain the dominant end-user segment for VADs, particularly in complex cases requiring sophisticated medical care.
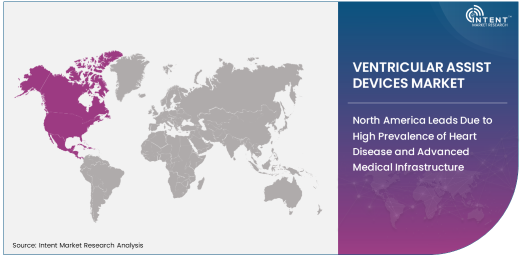
North America Leads Due to High Prevalence of Heart Disease and Advanced Medical Infrastructure
North America holds the largest share in the ventricular assist devices (VAD) market, primarily due to the high prevalence of heart failure and advanced healthcare systems that facilitate the adoption of these devices. The United States, being home to leading manufacturers and a substantial number of heart disease patients, is a key market. VADs are increasingly used as a bridge to heart transplant, and their growing use in long-term heart failure management has further fueled market growth.
The region’s well-established healthcare facilities and specialized cardiac care units, combined with ongoing research and development efforts, are essential drivers of the VAD market. Furthermore, the adoption of non-pulsatile flow devices in clinical settings is growing, leading to expanded market opportunities in North America.
Leading Companies and Competitive Landscape
Key players in the ventricular assist devices market include Abbott Laboratories, Medtronic, and Berlin Heart. These companies have a strong presence in the market, offering a broad range of VAD solutions, including LVADs, RVADs, and BiVADs. Abbott, for example, is a leader in the LVAD market with its HeartMate series, while Medtronic offers advanced VAD solutions that cater to a wide range of heart failure patients. The competition in this market is driven by continuous innovation, with companies focusing on improving the reliability, durability, and patient comfort of their devices.
The market also includes emerging players that are introducing next-generation devices and are focused on expanding into new geographies and developing new, cutting-edge technologies. These companies are increasingly investing in research and development to offer devices that provide longer-term circulatory support, lower risk of complications, and improved patient outcomes.
Recent Developments:
- In February 2025, Abbott Laboratories launched a new LVAD system designed to reduce complications and improve patient mobility post-implantation.
- In January 2025, Medtronic received FDA approval for its new biocompatible RVAD, marking a significant milestone in heart failure treatment options.
- In December 2024, Boston Scientific Corporation announced a partnership with a leading research university to develop next-generation VAD systems aimed at improving longevity and heart function.
- In November 2024, Berlin Heart GmbH expanded its market presence with the launch of an innovative Total Artificial Heart (TAH) solution for end-stage heart failure patients.
- In October 2024, SynCardia Systems received a significant investment to enhance the production of its portable VAD systems for emergency use.
List of Leading Companies:
- Abbott Laboratories
- Medtronic plc
- Boston Scientific Corporation
- Getinge AB
- SynCardia Systems, Inc.
- Terumo Corporation
- Berlin Heart GmbH
- Jarvik Heart, Inc.
- St. Jude Medical (acquired by Abbott)
- Cardiovascualr Systems, Inc.
- Ventricular Assist Devices Ltd.
- HeartWare International, Inc. (acquired by Medtronic)
- Thoratec Corporation (acquired by St. Jude Medical)
- MicroMed Cardiovascular, Inc.
- Carmat SA
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 1.2 billion |
|
Forecasted Value (2030) |
USD 2.5 billion |
|
CAGR (2025 – 2030) |
13.3% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Ventricular Assist Devices Market By Type (Left Ventricular Assist Devices (LVAD), Right Ventricular Assist Devices (RVAD), Biventricular Assist Devices (BiVAD), Total Artificial Heart (TAH)), By Flow Type (Pulsatile Flow Devices, Non-pulsatile Flow Devices), By End-User (Hospitals, Homecare, Specialized Heart Clinics) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Abbott Laboratories, Medtronic plc, Boston Scientific Corporation, Getinge AB, SynCardia Systems, Inc., Terumo Corporation, Berlin Heart GmbH, Jarvik Heart, Inc., St. Jude Medical (acquired by Abbott), Cardiovascualr Systems, Inc., Ventricular Assist Devices Ltd., HeartWare International, Inc. (acquired by Medtronic), Thoratec Corporation (acquired by St. Jude Medical), MicroMed Cardiovascular, Inc., Carmat SA |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Ventricular Assist Devices Market, by Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Left Ventricular Assist Devices (LVAD) |
|
4.2. Right Ventricular Assist Devices (RVAD) |
|
4.3. Biventricular Assist Devices (BiVAD) |
|
4.4. Total Artificial Heart (TAH) |
|
5. Ventricular Assist Devices Market, by Flow Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Pulsatile Flow Devices |
|
5.2. Non-pulsatile Flow Devices |
|
6. Ventricular Assist Devices Market, by End-User (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Hospitals |
|
6.2. Homecare |
|
6.3. Specialized Heart Clinics |
|
6.4. Others |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Ventricular Assist Devices Market, by Type |
|
7.2.7. North America Ventricular Assist Devices Market, by Flow Type |
|
7.2.8. North America Ventricular Assist Devices Market, by End-User |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Ventricular Assist Devices Market, by Type |
|
7.2.9.1.2. US Ventricular Assist Devices Market, by Flow Type |
|
7.2.9.1.3. US Ventricular Assist Devices Market, by End-User |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. Abbott Laboratories |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. Medtronic plc |
|
9.3. Boston Scientific Corporation |
|
9.4. Getinge AB |
|
9.5. SynCardia Systems, Inc. |
|
9.6. Terumo Corporation |
|
9.7. Berlin Heart GmbH |
|
9.8. Jarvik Heart, Inc. |
|
9.9. St. Jude Medical (acquired by Abbott) |
|
9.10. Cardiovascualr Systems, Inc. |
|
9.11. Ventricular Assist Devices Ltd. |
|
9.12. HeartWare International, Inc. (acquired by Medtronic) |
|
9.13. Thoratec Corporation (acquired by St. Jude Medical) |
|
9.14. MicroMed Cardiovascular, Inc. |
|
9.15. Carmat SA |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Ventricular Assist Devices Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Ventricular Assist Devices Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Ventricular Assist Devices Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
