As per Intent Market Research, the Varicose Vein Treatment Devices Market was valued at USD 0.7 billion in 2024-e and will surpass USD 1.6 billion by 2030; growing at a CAGR of 11.7% during 2025 - 2030.
The global varicose vein treatment devices market has witnessed significant growth driven by advancements in technology, increasing healthcare awareness, and rising incidences of vascular diseases. Varicose veins, characterized by swollen and twisted veins, often require medical intervention for relief and aesthetic purposes. The market for varicose vein treatment devices is primarily segmented by product type, application, end-user industry, and technology. The increasing demand for minimally invasive procedures is pushing the development of advanced devices that offer less recovery time and better patient outcomes. The market's growth trajectory is influenced by both technological innovation and the rising prevalence of varicose veins, particularly in aging populations and individuals with sedentary lifestyles.
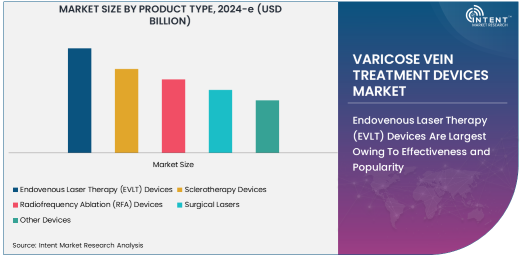
Endovenous Laser Therapy (EVLT) Devices Are Largest Owing To Effectiveness and Popularity
Among the various product types, Endovenous Laser Therapy (EVLT) devices dominate the market. This method uses laser energy to treat varicose veins by delivering heat through a catheter to close off the abnormal veins. EVLT devices are favored for their minimal invasiveness, faster recovery times, and high success rates. As patients increasingly prefer procedures with quicker recovery and fewer complications, the demand for EVLT has surged. Hospitals and clinics around the world are investing in these devices due to their proven efficiency in treating varicose veins, making them the most preferred choice for both doctors and patients.
The widespread use of EVLT is also fueled by the technological advancements in laser treatment technology. Newer EVLT systems offer more precise control, ensuring better outcomes for patients and minimizing side effects. The growing healthcare infrastructure in emerging markets also contributes to the sustained demand for EVLT devices.
Ambulatory Surgical Centers (ASCs) Are Fastest Growing Application Segment
In terms of application, Ambulatory Surgical Centers (ASCs) are the fastest-growing segment in the varicose vein treatment devices market. ASCs provide a convenient and cost-effective alternative to hospital-based procedures, offering patients the advantage of shorter wait times and lower costs for treatments. The shift toward outpatient care and the growing preference for less invasive treatments are driving the growth of ASCs. Additionally, with advances in medical technology, procedures like Endovenous Laser Therapy (EVLT) and Radiofrequency Ablation (RFA) are now more accessible and can be performed with minimal recovery time, making ASCs an attractive option for varicose vein treatments.
ASCs are also able to cater to a large number of patients efficiently, driving their rapid adoption. They are equipped with advanced treatment devices, providing an optimal environment for procedures that do not require overnight stays, contributing to their growing share in the market.
Healthcare Industry Is Largest End-User Industry Driving Market Demand
The healthcare industry is the largest end-user sector in the varicose vein treatment devices market. Healthcare providers, including hospitals and clinics, are at the forefront of adopting advanced varicose vein treatment devices due to their demand for high-quality, effective, and minimally invasive solutions. With an increasing focus on providing specialized vascular treatments and patient-centric care, the healthcare industry is heavily investing in varicose vein treatments to meet the growing needs of patients seeking relief from painful and unsightly varicose veins.
In particular, hospitals, with their capacity to handle a large number of cases and provide comprehensive medical support, remain the largest segment within this industry. Healthcare providers benefit from the latest devices to enhance the patient experience by offering quicker recovery times, fewer complications, and improved outcomes, reinforcing the market's growth.
Laser Treatment Technology Leads the Way in Varicose Vein Treatment Devices
Among the different technologies used for treating varicose veins, Laser Treatment Technology holds the largest share in the market. Laser treatments are particularly preferred due to their precision and minimal invasiveness. Laser energy, when applied to the affected veins, seals them off without the need for traditional surgery, resulting in faster recovery times and less discomfort for patients. The demand for laser-based solutions has risen as patients and healthcare providers seek non-invasive treatment options that offer faster results with fewer side effects.
Laser technology continues to evolve, with innovations aimed at improving treatment precision and reducing downtime. The popularity of laser treatment technology is expected to continue to rise as more healthcare facilities invest in these advanced, cost-effective, and highly efficient solutions.
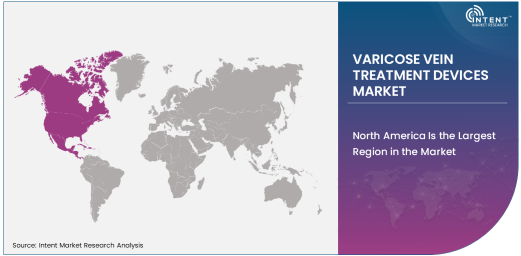
North America Is the Largest Region in the Market
In terms of geographical regions, North America is the largest market for varicose vein treatment devices, accounting for a substantial share of the global market. This is attributed to the high demand for advanced medical treatments, the presence of well-established healthcare infrastructure, and a high incidence of varicose veins, particularly among the aging population in countries like the United States and Canada.
Moreover, the region benefits from continuous innovation in medical devices, with several companies focusing on the development of state-of-the-art varicose vein treatment technologies. Government and private insurance schemes also support treatments for varicose veins, further fueling the demand for these devices. North America’s dominance is expected to continue, as patient awareness and technological advancements drive the market forward.
Leading Companies and Competitive Landscape
The varicose vein treatment devices market is highly competitive, with several leading companies continuously advancing their product portfolios to capture a larger market share. Major players such as Medtronic, Boston Scientific, and AngioDynamics dominate the market, offering a wide range of products, including Endovenous Laser Therapy (EVLT) devices, radiofrequency ablation (RFA) devices, and sclerotherapy devices. These companies are investing in R&D to introduce innovative products that provide better results, enhance patient comfort, and reduce recovery times.
The market also sees active mergers and acquisitions (M&A), as companies seek to expand their geographical reach and technological capabilities. With ongoing innovations in treatment options, especially related to laser and radiofrequency technology, the competition remains strong. Companies are focusing on strategic partnerships, technology advancements, and expanding distribution networks to stay ahead in this growing market
Recent Developments:
- Medtronic recently launched an advanced version of its Endovenous Laser Therapy (EVLT) system, offering improved precision for varicose vein treatments.
- AngioDynamics acquired Venclose, expanding its portfolio in the varicose vein treatment market, particularly for radiofrequency ablation devices.
- Boston Scientific received FDA approval for its Vascular Solutions Varicose Vein Treatment System, enhancing its competitive position in the market.
- Lumenis Ltd. introduced a new laser system for vein treatment that promises faster recovery times and more efficient results.
- Teleflex launched an updated version of its Radiofrequency Ablation (RFA) device, featuring improved ergonomics and energy delivery systems.
List of Leading Companies:
- Medtronic Plc
- Boston Scientific Corporation
- AngioDynamics Inc.
- Biolitec AG
- Cook Medical Inc.
- Dornier MedTech
- Lumenis Ltd.
- Vascular Solutions Inc.
- Abbott Laboratories
- Sciton Inc.
- Teleflex Inc.
- Merz Pharmaceuticals
- Smith & Nephew
- Cynosure Inc.
- VenClose LLC
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 0.7 Billion |
|
Forecasted Value (2030) |
USD 1.6 Billion |
|
CAGR (2025 – 2030) |
11.7% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Varicose Vein Treatment Devices Market By Product Type (Endovenous Laser Therapy (EVLT) Devices, Sclerotherapy Devices, Radiofrequency Ablation (RFA) Devices, Surgical Lasers), By Application (Hospitals, Clinics, Ambulatory Surgical Centers (ASCs), Home Healthcare), By End-User Industry (Healthcare, Medical Devices, Pharmaceuticals), and By Technology (Laser Treatment Technology, Radiofrequency Ablation Technology, Mechanical Compression Technology, Sclerotherapy Technology) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Medtronic Plc, Boston Scientific Corporation, AngioDynamics Inc., Biolitec AG, Cook Medical Inc., Dornier MedTech, Lumenis Ltd., Vascular Solutions Inc., Abbott Laboratories, Sciton Inc., Teleflex Inc., Merz Pharmaceuticals, Smith & Nephew, Cynosure Inc., VenClose LLC |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Varicose Vein Treatment Devices Market, by Product Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Endovenous Laser Therapy (EVLT) Devices |
|
4.2. Sclerotherapy Devices |
|
4.3. Radiofrequency Ablation (RFA) Devices |
|
4.4. Surgical Lasers |
|
4.5. Other Devices |
|
5. Varicose Vein Treatment Devices Market, by Application (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Hospitals |
|
5.2. Clinics |
|
5.3. Ambulatory Surgical Centers (ASCs) |
|
5.4. Home Healthcare |
|
6. Varicose Vein Treatment Devices Market, by End-User Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Healthcare |
|
6.2. Medical Devices |
|
6.3. Pharmaceutical |
|
6.4. Others |
|
7. Varicose Vein Treatment Devices Market, by Technology (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Laser Treatment Technology |
|
7.2. Radiofrequency Ablation Technology |
|
7.3. Mechanical Compression Technology |
|
7.4. Sclerotherapy Technology |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America Varicose Vein Treatment Devices Market, by Product Type |
|
8.2.7. North America Varicose Vein Treatment Devices Market, by Application |
|
8.2.8. North America Varicose Vein Treatment Devices Market, by End-User Industry |
|
8.2.9. North America Varicose Vein Treatment Devices Market, by Technology |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US Varicose Vein Treatment Devices Market, by Product Type |
|
8.2.10.1.2. US Varicose Vein Treatment Devices Market, by Application |
|
8.2.10.1.3. US Varicose Vein Treatment Devices Market, by End-User Industry |
|
8.2.10.1.4. US Varicose Vein Treatment Devices Market, by Technology |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Medtronic Plc |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Boston Scientific Corporation |
|
10.3. AngioDynamics Inc. |
|
10.4. Biolitec AG |
|
10.5. Cook Medical Inc. |
|
10.6. Dornier MedTech |
|
10.7. Lumenis Ltd. |
|
10.8. Vascular Solutions Inc. |
|
10.9. Abbott Laboratories |
|
10.10. Sciton Inc. |
|
10.11. Teleflex Inc. |
|
10.12. Merz Pharmaceuticals |
|
10.13. Smith & Nephew |
|
10.14. Cynosure Inc. |
|
10.15. VenClose LLC |
|
11. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Varicose Vein Treatment Devices Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Varicose Vein Treatment Devices Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Varicose Vein Treatment Devices Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
