As per Intent Market Research, the Safety Needles Market was valued at USD 6.1 billion in 2024-e and will surpass USD 9.4 billion by 2030; growing at a CAGR of 7.5% during 2025 - 2030.
The safety needles market has witnessed significant growth due to the increasing focus on healthcare worker safety and the prevention of needle stick injuries. Safety needles, designed to minimize accidental needle stick injuries, have become a crucial component in modern healthcare. Rising awareness about infection control, stringent regulatory requirements, and advancements in needle technology are driving the adoption of safety needles globally.
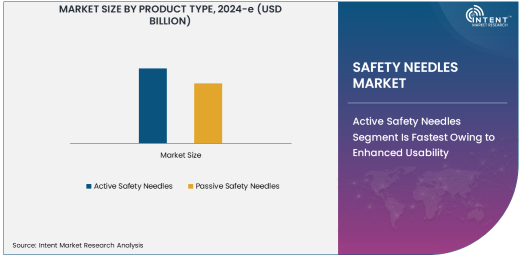
Active Safety Needles Segment Is Fastest Owing to Enhanced Usability
Active safety needles are experiencing rapid growth due to their user-friendly design and effectiveness in reducing needle stick injuries. These needles require manual activation of the safety mechanism, ensuring control during use. Their adoption is particularly high in regions with stringent safety regulations, such as North America and Europe.
The segment's growth is further fueled by healthcare facilities prioritizing safety and compliance with occupational health standards. Active safety needles are widely used in hospitals and clinics, where the volume of injections is high, enhancing their market dominance.
Hypodermic Needles Segment Is Largest Owing to Widespread Applications
Hypodermic needles hold the largest share in the application segment, attributed to their extensive use in delivering medications and vaccines. They are indispensable in healthcare settings, especially during vaccination drives and routine medical procedures.
The increasing prevalence of chronic diseases requiring frequent injections, such as diabetes and arthritis, has bolstered the demand for hypodermic safety needles. The COVID-19 pandemic further emphasized their importance, as they played a critical role in mass immunization programs globally.
Hospitals Segment Is Largest Owing to High Patient Influx
Hospitals dominate the end-user segment due to their high patient turnover and the need for large quantities of safety needles for various medical procedures. These facilities are the primary consumers of safety needles, driven by stringent safety protocols and infection prevention measures.
The rising number of surgeries, increasing prevalence of chronic illnesses, and growing investments in healthcare infrastructure are propelling the demand for safety needles in hospitals. Additionally, hospitals' focus on adopting advanced safety devices to safeguard healthcare workers has reinforced the segment's growth.
Online Pharmacies Segment Is Fastest Owing to E-Commerce Expansion
The online pharmacies segment is witnessing rapid growth, driven by the increasing penetration of e-commerce in the healthcare sector. Patients and healthcare providers are opting for online platforms due to convenience, competitive pricing, and accessibility to a broad range of products.
This growth is particularly evident in emerging markets where digital healthcare platforms are gaining traction. The COVID-19 pandemic accelerated this trend, as online pharmacies became a preferred channel for procuring medical supplies, including safety needles.
North America Is Largest Owing to Advanced Healthcare Infrastructure
North America leads the safety needles market, primarily due to its advanced healthcare infrastructure, high awareness about needle safety, and strict regulatory landscape. The region's focus on reducing healthcare-associated infections (HAIs) and ensuring worker safety has driven the adoption of safety needles.
The presence of key market players and ongoing technological advancements further strengthen North America's position as the largest regional market. Additionally, extensive vaccination campaigns and increasing prevalence of chronic diseases contribute to sustained growth.

Competitive Landscape and Leading Companies
The safety needles market is highly competitive, with major players focusing on innovation and strategic collaborations to enhance their market presence. Companies such as Becton, Dickinson and Company (BD), Smiths Medical, and Terumo Corporation lead the market with extensive product portfolios and global distribution networks.
The competitive landscape is marked by product launches, mergers and acquisitions, and partnerships aimed at expanding geographic reach and technological capabilities. For instance, advancements in retractable and passive safety needle technologies are reshaping the market, ensuring safer and more efficient injection practices.
Recent Developments:
- Becton, Dickinson and Company (BD) launched a new range of retractable safety syringes in October 2024, enhancing its product portfolio.
- Medtronic plc announced the acquisition of a needle safety device startup to integrate advanced needle stick prevention technologies.
- Terumo Corporation unveiled its new line of passive safety needles, focusing on user convenience and compliance with global safety standards.
- Smiths Medical secured regulatory approval for its advanced hypodermic safety needles in the European market in September 2024.
- Cardinal Health partnered with a major distributor to expand its safety needle offerings in the Asia-Pacific region.
List of Leading Companies:
- Becton, Dickinson and Company (BD)
- Smiths Medical
- Terumo Corporation
- Medtronic plc
- Nipro Corporation
- Cardinal Health
- B. Braun Melsungen AG
- Novo Nordisk A/S
- Retractable Technologies, Inc.
- Greiner Bio-One International GmbH
- Vygon SA
- Merit Medical Systems
- Teleflex Incorporated
- Allison Medical, Inc.
- Hamilton Company
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 6.1 Billion |
|
Forecasted Value (2030) |
USD 9.4 Billion |
|
CAGR (2025 – 2030) |
7.5% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Safety Needles Market By Product Type (Active Safety Needles, Passive Safety Needles), By Application (Hypodermic Needles, Insulin Needles, Blood Collection Needles, Spinal Needles, Intravenous (IV) Needles), By End-User (Hospitals, Clinics, Ambulatory Surgical Centers (ASCs), Home Care Settings), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Becton, Dickinson and Company (BD), Smiths Medical, Terumo Corporation, Medtronic plc, Nipro Corporation, Cardinal Health, B. Braun Melsungen AG, Novo Nordisk A/S, Retractable Technologies, Inc., Greiner Bio-One International GmbH, Vygon SA, Merit Medical Systems, Teleflex Incorporated, Allison Medical, Inc., Hamilton Company |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Safety Needles Market, by Product Type (Market Size & Forecast: USD Million, 2022 – 2030) |
|
4.1. Active Safety Needles |
|
4.2. Passive Safety Needles |
|
5. Safety Needles Market, by Application (Market Size & Forecast: USD Million, 2022 – 2030) |
|
5.1. Hypodermic Needles |
|
5.2. Insulin Needles |
|
5.3. Blood Collection Needles |
|
5.4. Spinal Needles |
|
5.5. Intravenous (IV) Needles |
|
6. Safety Needles Market, by End-User (Market Size & Forecast: USD Million, 2022 – 2030) |
|
6.1. Hospitals |
|
6.2. Clinics |
|
6.3. Ambulatory Surgical Centers (ASCs) |
|
6.4. Home Care Settings |
|
7. Safety Needles Market, by Distribution Channel (Market Size & Forecast: USD Million, 2022 – 2030) |
|
7.1. Hospital Pharmacies |
|
7.2. Retail Pharmacies |
|
7.3. Online Pharmacies |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2022 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America Safety Needles Market, by Product Type |
|
8.2.7. North America Safety Needles Market, by Application |
|
8.2.8. North America Safety Needles Market, by End-User |
|
8.2.9. North America Safety Needles Market, by Distribution Channel |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US Safety Needles Market, by Product Type |
|
8.2.10.1.2. US Safety Needles Market, by Application |
|
8.2.10.1.3. US Safety Needles Market, by End-User |
|
8.2.10.1.4. US Safety Needles Market, by Distribution Channel |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Becton, Dickinson and Company (BD) |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Smiths Medical |
|
10.3. Terumo Corporation |
|
10.4. Medtronic plc |
|
10.5. Nipro Corporation |
|
10.6. Cardinal Health |
|
10.7. B. Braun Melsungen AG |
|
10.8. Novo Nordisk A/S |
|
10.9. Retractable Technologies, Inc. |
|
10.10. Greiner Bio-One International GmbH |
|
10.11. Vygon SA |
|
10.12. Merit Medical Systems |
|
10.13. Teleflex Incorporated |
|
10.14. Allison Medical, Inc. |
|
10.15. Hamilton Company |
|
11. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Safety Needles Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Safety Needles Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Safety Needles Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
