As per Intent Market Research, the Pharmaceutical Unit-Dose Packaging Market was valued at USD 3.2 Billion in 2024-e and will surpass USD 5.7 Billion by 2030; growing at a CAGR of 10.0% during 2025 - 2030.
The pharmaceutical unit-dose packaging market plays a critical role in ensuring the safe, efficient, and accurate delivery of medications. Unit-dose packaging is designed to hold a single dose of medication, allowing for easy administration while minimizing the risk of dosing errors. The growing demand for unit-dose packaging stems from the increasing focus on patient safety, medication adherence, and the need for streamlined drug distribution in hospitals, clinics, and pharmacies. This packaging method also facilitates the handling of a variety of drug forms, such as tablets, capsules, and liquids, while offering benefits like improved shelf-life, contamination prevention, and tamper-evidence. The market is further fueled by innovations in packaging materials and the ongoing adoption of sustainable and user-friendly solutions.
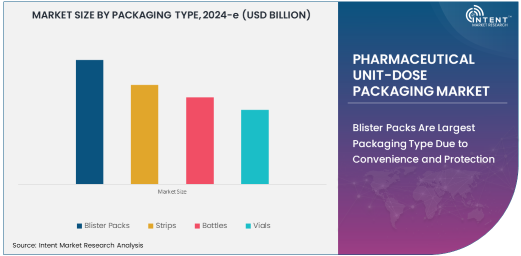
Blister Packs Are Largest Packaging Type Due to Convenience and Protection
Blister packs are the largest packaging type in the pharmaceutical unit-dose packaging market, owing to their widespread use in the packaging of tablets and capsules. These packs offer several benefits, including superior protection against moisture, light, and contamination, making them ideal for pharmaceutical products that require extended shelf-life. The transparent plastic covering in blister packs also allows patients and healthcare professionals to easily identify individual doses, enhancing convenience and medication adherence.
Blister packs are particularly popular in the packaging of over-the-counter drugs and prescription medications. Their compact and tamper-evident nature makes them highly suitable for pharmaceutical packaging, where ensuring product integrity and patient safety is paramount. Furthermore, the ease of use, combined with cost-effectiveness and customization options, contributes to the growing demand for blister packs in the pharmaceutical industry.
Plastic Is Largest Material Type Due to Cost-Effectiveness and Versatility
Plastic is the largest material type used in pharmaceutical unit-dose packaging due to its cost-effectiveness, flexibility, and ability to provide adequate protection for drugs. Plastic materials, such as polyvinyl chloride (PVC) and polyethylene, are widely used for blister packs, bottles, and vials, as they offer the necessary protection from moisture, air, and physical damage while maintaining product integrity. The versatility of plastic also enables packaging solutions that are lightweight, durable, and easy to manufacture in various shapes and sizes.
The increasing demand for sustainable packaging has prompted innovation in the plastic materials used for unit-dose packaging, with a focus on biodegradable and recyclable options. Plastic’s combination of affordability, durability, and adaptability makes it the preferred material for pharmaceutical packaging, especially for large-scale drug manufacturing and distribution.
Pharmaceutical Manufacturers Are Largest End-Use Industry Due to Growing Demand for Drug Packaging Solutions
Pharmaceutical manufacturers represent the largest end-use industry in the unit-dose packaging market. The growing need for pharmaceutical products, especially in developing countries, coupled with the rising emphasis on safe and efficient medication delivery, is driving the demand for unit-dose packaging solutions. Pharmaceutical manufacturers rely on unit-dose packaging to ensure their products are delivered safely and accurately to patients, reducing the risk of medication errors.
With increasing regulatory requirements around product safety, traceability, and patient compliance, pharmaceutical manufacturers are investing in innovative packaging technologies. These technologies, including tamper-evident and child-resistant packaging, provide additional security and ease of use. As pharmaceutical companies continue to prioritize packaging that enhances patient experience and reduces the risk of contamination, the demand for pharmaceutical unit-dose packaging solutions is expected to grow steadily.
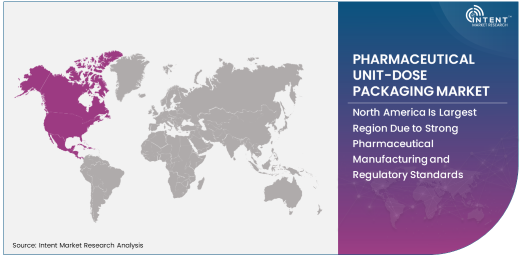
North America Is Largest Region Due to Strong Pharmaceutical Manufacturing and Regulatory Standards
North America is the largest region in the pharmaceutical unit-dose packaging market, driven by the robust pharmaceutical manufacturing industry, advanced healthcare infrastructure, and strict regulatory standards in the region. The U.S., in particular, plays a significant role in driving the demand for pharmaceutical unit-dose packaging due to its large pharmaceutical market, a strong focus on patient safety, and the continuous evolution of packaging technologies.
Additionally, the high prevalence of chronic diseases and the growing demand for both prescription and over-the-counter medications contribute to the region’s market leadership. Regulatory bodies such as the FDA also enforce strict guidelines regarding packaging, which further emphasizes the need for secure and efficient unit-dose packaging solutions. The North American market is expected to remain dominant, fueled by ongoing innovations in packaging and an increased focus on healthcare quality and patient safety.
Competitive Landscape and Key Players
The pharmaceutical unit-dose packaging market is competitive, with leading players such as Amcor, West Pharmaceutical Services, and Uhlmann Packaging Systems shaping the industry. These companies offer a range of packaging solutions that cater to various pharmaceutical needs, from blister packs to bottles and vials, and serve multiple industries including pharmaceutical manufacturers, contract packaging companies, and healthcare providers.
To remain competitive, companies in this market focus on enhancing packaging efficiency, reducing costs, and ensuring compliance with global regulatory standards. Innovations in sustainable packaging materials and technologies are also key strategies for these players. The rise of patient-centric packaging, such as easy-to-open blister packs and child-resistant options, is becoming a priority in response to growing concerns about medication adherence and safety. Strategic collaborations, mergers, and acquisitions are common in the market as companies seek to expand their capabilities and market reach.
Recent Developments:
- Amcor Limited introduced a new line of recyclable unit-dose blister packaging, aiming to reduce the environmental footprint of pharmaceutical packaging.
- West Pharmaceutical Services launched an innovative unit-dose packaging solution designed to enhance the stability and safety of biologic drugs.
- SCHOTT AG unveiled a new glass vial packaging solution, offering improved protection for sensitive pharmaceuticals while maintaining ease of use for healthcare providers.
- Gerresheimer AG expanded its unit-dose packaging portfolio, focusing on the development of sustainable packaging solutions for injectable drugs.
- AptarGroup, Inc. announced the release of a next-gen unit-dose packaging system designed to provide better integration with drug delivery devices, enhancing patient experience and adherence.
List of Leading Companies:
- Amcor Limited
- Becton, Dickinson and Company (BD)
- West Pharmaceutical Services, Inc.
- Capsa Solutions
- Rondo Ganahl AG
- Uhlmann Pac-Systeme GmbH & Co. KG
- SCHOTT AG
- Gerresheimer AG
- AptarGroup, Inc.
- Huhtamaki Group
- Baxter International Inc.
- Mylan N.V. (Viatris Inc.)
- SteriPack Group
- Drug Plastics & Glass Company, Inc.
- Portola Packaging, Inc.
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 3.2 Billion |
|
Forecasted Value (2030) |
USD 5.7 Billion |
|
CAGR (2025 – 2030) |
10.0% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Global Pharmaceutical Unit-Dose Packaging Market by Packaging Type (Blister Packs, Strips, Bottles, Vials), by Material Type (Plastic, Glass, Aluminum, Paper), by End-Use Industry (Pharmaceutical Manufacturers, Contract Packaging Companies, Healthcare Providers); Insights & Forecast (2024 – 2030) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Amcor Limited, Becton, Dickinson and Company (BD), West Pharmaceutical Services, Inc., Capsa Solutions, Rondo Ganahl AG, Uhlmann Pac-Systeme GmbH & Co. KG, Gerresheimer AG, AptarGroup, Inc., Huhtamaki Group, Baxter International Inc., Mylan N.V. (Viatris Inc.), SteriPack Group, Portola Packaging, Inc. |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Pharmaceutical Unit-Dose Packaging Market, by Packaging Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Blister Packs |
|
4.2. Strips |
|
4.3. Bottles |
|
4.4. Vials |
|
5. Pharmaceutical Unit-Dose Packaging Market, by Material Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Plastic |
|
5.2. Glass |
|
5.3. Aluminum |
|
5.4. Paper |
|
6. Pharmaceutical Unit-Dose Packaging Market, by End-Use Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Pharmaceutical Manufacturers |
|
6.2. Contract Packaging Companies |
|
6.3. Healthcare Providers |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Pharmaceutical Unit-Dose Packaging Market, by Packaging Type |
|
7.2.7. North America Pharmaceutical Unit-Dose Packaging Market, by Material Type |
|
7.2.8. North America Pharmaceutical Unit-Dose Packaging Market, by End-Use Industry |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Pharmaceutical Unit-Dose Packaging Market, by Packaging Type |
|
7.2.9.1.2. US Pharmaceutical Unit-Dose Packaging Market, by Material Type |
|
7.2.9.1.3. US Pharmaceutical Unit-Dose Packaging Market, by End-Use Industry |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. Amcor Limited |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. Becton, Dickinson and Company (BD) |
|
9.3. West Pharmaceutical Services, Inc. |
|
9.4. Capsa Solutions |
|
9.5. Rondo Ganahl AG |
|
9.6. Uhlmann Pac-Systeme GmbH & Co. KG |
|
9.7. SCHOTT AG |
|
9.8. Gerresheimer AG |
|
9.9. AptarGroup, Inc. |
|
9.10. Huhtamaki Group |
|
9.11. Baxter International Inc. |
|
9.12. Mylan N.V. (Viatris Inc.) |
|
9.13. SteriPack Group |
|
9.14. Drug Plastics & Glass Company, Inc. |
|
9.15. Portola Packaging, Inc. |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Pharmaceutical Unit-Dose Packaging Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Pharmaceutical Unit-Dose Packaging Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Pharmaceutical Unit-Dose Packaging Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
