As per Intent Market Research, the Pharmaceutical Quality Control Market was valued at USD 5.2 Billion in 2024-e and will surpass USD 9.1 Billion by 2030; growing at a CAGR of 9.9% during 2025 - 2030.
The pharmaceutical quality control (QC) market plays a crucial role in ensuring the safety, efficacy, and compliance of pharmaceutical products throughout the manufacturing process. With strict regulatory requirements and increasing demand for high-quality medications, pharmaceutical QC testing has become more sophisticated, encompassing various testing types such as analytical, microbiological, and chemical testing. Quality control is pivotal in preventing product defects and ensuring that drugs meet the standards of safety, stability, and performance required by regulatory authorities.
The pharmaceutical QC market is expected to continue its growth, driven by the rising emphasis on maintaining high standards of quality assurance, the launch of new drugs, and the growing adoption of advanced technologies for more accurate testing. The market’s expansion is also supported by the increasing demand for contract manufacturing services and the continued focus on research and development (R&D) activities aimed at enhancing drug formulations and delivery mechanisms.
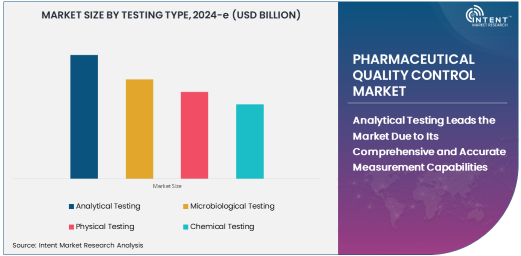
Analytical Testing Leads the Market Due to Its Comprehensive and Accurate Measurement Capabilities
Analytical testing is the largest testing type in the pharmaceutical quality control market, driven by its ability to provide precise and comprehensive data on the composition, purity, and stability of pharmaceutical products. Analytical tests are essential for ensuring that raw materials, in-process samples, and finished products meet the stringent quality standards set by regulatory bodies. Techniques such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and spectroscopy are frequently used in analytical testing to determine the chemical structure, content, and potential impurities in pharmaceutical formulations.
The need for analytical testing has grown due to the increasing complexity of modern drug formulations, particularly biologics and gene therapies, which require highly sensitive testing methods. This type of testing provides critical insights into the performance of pharmaceutical products and ensures their safety for consumers. The growing number of new drug approvals and the complexity of formulations are expected to drive further demand for analytical testing services in the pharmaceutical QC market.
Finished Product Testing Drives Demand for Comprehensive Quality Assurance
Finished product testing is the largest test category in the pharmaceutical quality control market, as it is essential to ensure that final pharmaceutical products meet the required quality standards before they are released to the market. This category of testing includes a broad range of assessments, including potency, stability, packaging integrity, and compliance with regulatory specifications. Finished product testing is critical in identifying defects that may arise during production and ensuring that the product delivers the intended therapeutic effect to the patient.
Finished product testing is also important for maintaining consistency in drug efficacy and safety across different production batches. As the pharmaceutical industry increasingly shifts toward personalized medicine and complex formulations, the need for robust and accurate finished product testing will continue to grow. This trend is expected to drive the expansion of the finished product testing segment in the pharmaceutical QC market.
Pharmaceutical Manufacturers Lead the End-Use Industry Due to High Quality Assurance Requirements
Pharmaceutical manufacturers are the largest end-use industry for the pharmaceutical quality control market, as they are directly involved in the production of pharmaceutical products that require rigorous testing to ensure quality and compliance with regulatory standards. These manufacturers rely on a variety of QC tests throughout the production process to ensure that their products are safe, effective, and of the highest quality.
The increasing pressure to meet regulatory requirements, maintain high manufacturing standards, and reduce the risk of product recalls has led to a greater focus on quality control in the pharmaceutical manufacturing sector. As a result, pharmaceutical manufacturers are investing heavily in advanced testing technologies and services to ensure that their products meet the necessary standards for approval and consumer safety. This demand is expected to continue driving the pharmaceutical QC market.
North America Leads the Market Due to Strict Regulatory Standards and Advanced Testing Technologies
North America is the largest region in the pharmaceutical quality control market, driven by stringent regulatory standards set by authorities such as the U.S. Food and Drug Administration (FDA) and Health Canada. These regulations mandate comprehensive quality control testing to ensure that pharmaceutical products meet safety, efficacy, and quality requirements. The region also benefits from advanced technological capabilities, such as automated testing systems and state-of-the-art laboratories, which improve the efficiency and accuracy of QC testing processes.
The presence of major pharmaceutical companies, contract research organizations (CROs), and contract manufacturing organizations (CMOs) in North America further supports the growth of the QC market. Additionally, the ongoing development of new drug formulations, including biologics and biosimilars, is expected to drive demand for more advanced testing services, boosting the pharmaceutical QC market in the region.

Competitive Landscape and Key Players
The pharmaceutical quality control market is highly competitive, with several global and regional players offering a variety of testing services and technologies. Key players in the market include Thermo Fisher Scientific, Charles River Laboratories, Lonza Group, IQVIA, and WuXi AppTec. These companies are focused on expanding their portfolios through strategic acquisitions, partnerships, and investments in R&D to meet the growing demand for quality control services.
The competitive landscape is also shaped by the increasing need for specialized testing services for emerging drug types, such as gene therapies and biologics, as well as the adoption of automation and AI technologies in testing processes. Companies are investing in advanced solutions to enhance the accuracy, efficiency, and scalability of their QC testing services, enabling them to meet the evolving needs of pharmaceutical manufacturers and regulatory authorities.
Recent Developments:
- Thermo Fisher Scientific Inc. launched a new quality control testing solution aimed at improving the accuracy and speed of pharmaceutical product testing.
- Agilent Technologies Inc. announced a partnership with a leading pharmaceutical company to enhance the quality control process using advanced analytical tools.
- Merck Group unveiled an innovative testing platform for faster and more reliable quality control in biologics manufacturing.
- Waters Corporation introduced a new chromatography system designed for efficient pharmaceutical quality testing and regulatory compliance.
- Danaher Corporation acquired a laboratory automation company, expanding its capabilities in pharmaceutical quality control testing and sample management.
List of Leading Companies:
- Thermo Fisher Scientific Inc.
- Agilent Technologies Inc.
- PerkinElmer Inc.
- Bio-Rad Laboratories Inc.
- Merck Group
- Abbott Laboratories
- Siemens Healthineers AG
- Becton, Dickinson and Company
- F. Hoffmann-La Roche Ltd.
- Danaher Corporation
- Waters Corporation
- Charles River Laboratories International, Inc.
- Eppendorf AG
- Shimadzu Corporation
- Lonza Group
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 5.2 Billion |
|
Forecasted Value (2030) |
USD 9.1 Billion |
|
CAGR (2025 – 2030) |
9.9% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Global Pharmaceutical Quality Control Market by Testing Type (Analytical Testing, Microbiological Testing, Physical Testing, Chemical Testing), by Test Category (Raw Material Testing, In-Process Testing, Finished Product Testing), by End-Use Industry (Pharmaceutical Manufacturers, Contract Research Organizations (CROs), Contract Manufacturing Organizations (CMOs), Research and Development (R&D)); Insights & Forecast (2024 – 2030) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Thermo Fisher Scientific Inc., Agilent Technologies Inc., PerkinElmer Inc., Bio-Rad Laboratories Inc., Merck Group, Abbott Laboratories, Becton, Dickinson and Company, F. Hoffmann-La Roche Ltd., Danaher Corporation, Waters Corporation, Charles River Laboratories International, Inc., Eppendorf AG, Lonza Group |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Pharmaceutical Quality Control Market, by Testing Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Analytical Testing |
|
4.2. Microbiological Testing |
|
4.3. Physical Testing |
|
4.4. Chemical Testing |
|
5. Pharmaceutical Quality Control Market, by Test Category (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Raw Material Testing |
|
5.2. In-Process Testing |
|
5.3. Finished Product Testing |
|
6. Pharmaceutical Quality Control Market, by End-Use Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Pharmaceutical Manufacturers |
|
6.2. Contract Research Organizations (CROs) |
|
6.3. Contract Manufacturing Organizations (CMOs) |
|
6.4. Research and Development (R&D) |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Pharmaceutical Quality Control Market, by Testing Type |
|
7.2.7. North America Pharmaceutical Quality Control Market, by Test Category |
|
7.2.8. North America Pharmaceutical Quality Control Market, by End-Use Industry |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Pharmaceutical Quality Control Market, by Testing Type |
|
7.2.9.1.2. US Pharmaceutical Quality Control Market, by Test Category |
|
7.2.9.1.3. US Pharmaceutical Quality Control Market, by End-Use Industry |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. Thermo Fisher Scientific Inc. |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. Agilent Technologies Inc. |
|
9.3. PerkinElmer Inc. |
|
9.4. Bio-Rad Laboratories Inc. |
|
9.5. Merck Group |
|
9.6. Abbott Laboratories |
|
9.7. Siemens Healthineers AG |
|
9.8. Becton, Dickinson and Company |
|
9.9. F. Hoffmann-La Roche Ltd. |
|
9.10. Danaher Corporation |
|
9.11. Waters Corporation |
|
9.12. Charles River Laboratories International, Inc. |
|
9.13. Eppendorf AG |
|
9.14. Shimadzu Corporation |
|
9.15. Lonza Group |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Pharmaceutical Quality Control Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Pharmaceutical Quality Control Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Pharmaceutical Quality Control Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
