As per Intent Market Research, the Multi-Parameter Patient Monitoring Equipment Market was valued at USD 3.1 Billion in 2024-e and will surpass USD 5.0 Billion by 2030; growing at a CAGR of 8.6% during 2025 - 2030.
The multi-parameter patient monitoring equipment market is witnessing robust growth, driven by the increasing demand for continuous monitoring of patients' vital signs, particularly in critical and emergency care settings. These monitoring systems are used to track multiple physiological parameters such as heart rate, blood pressure, respiratory rate, and temperature, offering a comprehensive view of a patient's health status. The market is expanding with technological advancements, which are leading to the development of more portable, user-friendly, and efficient monitoring solutions. These devices are essential in various healthcare settings, including hospitals, clinics, home healthcare, and ambulatory surgical centers, where patient safety and timely intervention are critical.
The demand for multi-parameter patient monitoring equipment is increasing due to the rising prevalence of chronic diseases, an aging global population, and growing healthcare infrastructure. Innovations in monitoring technologies, such as wireless connectivity, cloud-based solutions, and integration with electronic health records (EHR), are further fueling market growth. The shift towards patient-centered care, which emphasizes early diagnosis and real-time monitoring, is creating new opportunities for the market. With increasing awareness regarding the importance of continuous patient monitoring, the multi-parameter patient monitoring equipment market is expected to expand further in the coming years.
Bedside Monitors Are Largest Owing to Wide Adoption in Hospitals and Clinics
Bedside monitors hold the largest market share in the multi-parameter patient monitoring equipment sector, primarily due to their extensive use in hospitals and clinics. These devices are designed to continuously monitor patients' vital signs in real-time, especially in critical care, emergency, and intensive care units (ICUs). Bedside monitors provide healthcare professionals with immediate and accurate information, enabling quick decision-making and timely interventions. Their ability to track multiple parameters simultaneously, such as heart rate, respiratory rate, blood pressure, oxygen saturation, and temperature, makes them indispensable in high-acuity care environments.
The demand for bedside monitors is driven by the need for continuous patient surveillance in hospitals, especially for patients undergoing major surgeries, in the ICU, or in emergency care units. These monitors are known for their reliability, ease of use, and integration with hospital information systems, making them a preferred choice for healthcare providers. As hospitals continue to focus on improving patient outcomes and reducing healthcare-associated risks, the use of bedside monitors is expected to remain dominant in the multi-parameter patient monitoring equipment market.
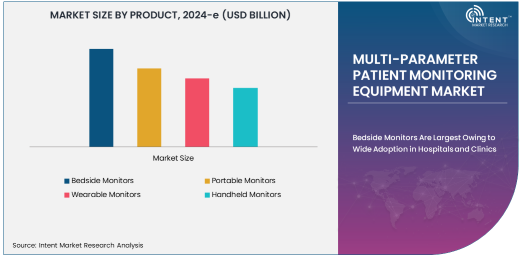
Portable Monitors Are Fastest Growing Owing to Increasing Demand for Home Healthcare
The portable monitors segment is the fastest growing in the multi-parameter patient monitoring equipment market, driven by the rising demand for home healthcare solutions and the increasing focus on remote patient monitoring. Portable monitors offer the convenience of mobility, allowing patients to track their vital signs at home or while on the go. These devices are especially beneficial for individuals with chronic conditions, such as heart disease, diabetes, and respiratory disorders, who require continuous monitoring but prefer to manage their health outside of a hospital setting.
As the healthcare industry shifts towards outpatient and home-based care, portable monitors are gaining popularity due to their compact size, ease of use, and ability to connect with mobile apps or healthcare providers' networks. This trend is supported by advancements in wireless technology, which allow patients to transmit their data in real-time to healthcare providers, enabling proactive management of their conditions. The growing emphasis on reducing hospital readmissions and the increasing preference for home healthcare will continue to drive the rapid adoption of portable monitors in the market.
Hospitals and Clinics Are Largest End-Use Industry Due to High Demand for Continuous Monitoring
The hospitals and clinics segment is the largest end-use industry for multi-parameter patient monitoring equipment, owing to the high demand for continuous and real-time monitoring of patients, especially in critical care settings. Hospitals and clinics rely on multi-parameter monitors to track patients' vital signs and ensure timely medical intervention. The increasing number of surgeries, emergency cases, and the rising prevalence of chronic conditions are contributing to the widespread use of these devices in healthcare institutions.
Hospitals, particularly those with intensive care units (ICUs), emergency rooms (ERs), and operating theaters, rely on multi-parameter patient monitoring equipment to maintain the safety and health of their patients. The integration of advanced features such as alarm systems, connectivity with health records, and data analytics into these monitoring devices further enhances their functionality and value. As hospitals continue to invest in advanced technologies to improve patient care and outcomes, the demand for multi-parameter patient monitoring equipment in these settings is expected to remain high.
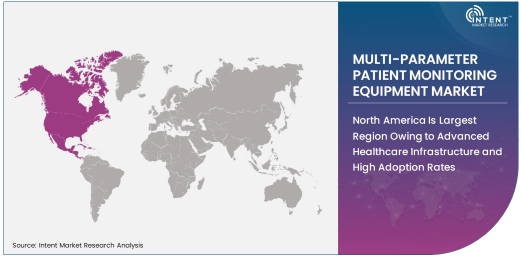
North America Is Largest Region Owing to Advanced Healthcare Infrastructure and High Adoption Rates
North America remains the largest region in the multi-parameter patient monitoring equipment market, driven by its advanced healthcare infrastructure, high healthcare spending, and early adoption of new medical technologies. The United States, in particular, is a key market, where healthcare providers are increasingly adopting state-of-the-art monitoring systems to enhance patient care and ensure better outcomes. The growing incidence of chronic diseases, such as cardiovascular disease, diabetes, and respiratory disorders, has further spurred the demand for continuous patient monitoring in hospitals, clinics, and home healthcare settings.
North America's robust healthcare infrastructure and emphasis on patient safety have led to widespread adoption of multi-parameter patient monitoring systems across various healthcare institutions. The region also benefits from a strong presence of leading medical device manufacturers and a favorable regulatory environment that ensures the safety and efficacy of medical devices. As healthcare systems continue to focus on improving patient outcomes and reducing healthcare costs, North America is expected to maintain its dominance in the multi-parameter patient monitoring equipment market.
Leading Companies and Competitive Landscape
The multi-parameter patient monitoring equipment market is highly competitive, with several prominent players offering a variety of solutions designed to meet the needs of healthcare providers. Leading companies in the market include Philips Healthcare, Siemens Healthineers, GE Healthcare, Medtronic, and Mindray. These companies offer a wide range of monitoring devices, including bedside monitors, portable monitors, and wearable devices, which are equipped with advanced features such as wireless connectivity, cloud integration, and real-time data analytics.
The competitive landscape is characterized by continuous product innovation and technological advancements, with companies focusing on enhancing the functionality, portability, and user-friendliness of their monitoring equipment. Additionally, mergers and acquisitions, partnerships, and collaborations are common strategies adopted by leading players to expand their market presence and develop cutting-edge solutions. As the demand for remote monitoring and home healthcare solutions grows, the market for multi-parameter patient monitoring equipment will continue to evolve, with companies striving to meet the ever-changing needs of the healthcare industry.
Recent Developments:
- Philips Healthcare launched a new wearable multi-parameter monitoring solution in 2024 that integrates advanced analytics for real-time clinical decision support.
- GE Healthcare introduced a new generation of portable multi-parameter monitors designed for use in ambulatory surgical centers, improving patient mobility and monitoring accuracy.
- Medtronic plc expanded its patient monitoring portfolio by acquiring a leading telemetry monitoring company, enhancing its presence in home healthcare monitoring.
- Nihon Kohden Corporation unveiled a state-of-the-art multi-parameter monitor with advanced ECG technology, improving accuracy for cardiovascular monitoring in critical care settings.
- Mindray Medical International Limited launched a new bedside multi-parameter monitoring system equipped with cloud-based data integration, enabling remote patient monitoring and collaboration.
List of Leading Companies:
- Philips Healthcare
- GE Healthcare
- Siemens Healthineers
- Medtronic plc
- Abbott Laboratories
- Honeywell Life Care Solutions
- Nihon Kohden Corporation
- Schiller AG
- Drägerwerk AG & Co. KGaA
- Osso VR
- Mindray Medical International Limited
- Roche Diagnostics
- Fujifilm Holdings Corporation
- Ampronix
- CAS Medical Systems, Inc.
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 3.1 Billion |
|
Forecasted Value (2030) |
USD 5.0 Billion |
|
CAGR (2025 – 2030) |
8.6% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Global Multi-Parameter Patient Monitoring Equipment Market by Product (Bedside Monitors, Portable Monitors, Wearable Monitors, Handheld Monitors), Application (Cardiovascular Monitoring, Respiratory Monitoring, Neurological Monitoring), End-Use Industry (Hospitals and Clinics, Home Healthcare, Ambulatory Surgical Centers) and Regions |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Philips Healthcare, GE Healthcare, Siemens Healthineers, Medtronic plc, Abbott Laboratories, Honeywell Life Care Solutions, Schiller AG, Drägerwerk AG & Co. KGaA, Osso VR, Mindray Medical International Limited, Roche Diagnostics, Fujifilm Holdings Corporation, CAS Medical Systems, Inc.
|
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Multi-Parameter Patient Monitoring Equipment Market, by Product (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Bedside Monitors |
|
4.2. Portable Monitors |
|
4.3. Wearable Monitors |
|
4.4. Handheld Monitors |
|
5. Multi-Parameter Patient Monitoring Equipment Market, by Application (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Cardiovascular Monitoring |
|
5.2. Respiratory Monitoring |
|
5.3. Neurological Monitoring |
|
5.4. Others |
|
6. Multi-Parameter Patient Monitoring Equipment Market, by End-Use Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Hospitals and Clinics |
|
6.2. Home Healthcare |
|
6.3. Ambulatory Surgical Centers |
|
6.4. Others |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Multi-Parameter Patient Monitoring Equipment Market, by Product |
|
7.2.7. North America Multi-Parameter Patient Monitoring Equipment Market, by Application |
|
7.2.8. North America Multi-Parameter Patient Monitoring Equipment Market, by End-Use Industry |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Multi-Parameter Patient Monitoring Equipment Market, by Product |
|
7.2.9.1.2. US Multi-Parameter Patient Monitoring Equipment Market, by Application |
|
7.2.9.1.3. US Multi-Parameter Patient Monitoring Equipment Market, by End-Use Industry |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. Philips Healthcare |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. GE Healthcare |
|
9.3. Siemens Healthineers |
|
9.4. Medtronic plc |
|
9.5. Abbott Laboratories |
|
9.6. Honeywell Life Care Solutions |
|
9.7. Nihon Kohden Corporation |
|
9.8. Schiller AG |
|
9.9. Drägerwerk AG & Co. KGaA |
|
9.10. Osso VR |
|
9.11. Mindray Medical International Limited |
|
9.12. Roche Diagnostics |
|
9.13. Fujifilm Holdings Corporation |
|
9.14. Ampronix |
|
9.15. CAS Medical Systems, Inc. |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Multi-Parameter Patient Monitoring Equipment Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Multi-Parameter Patient Monitoring Equipment Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Multi-Parameter Patient Monitoring Equipment Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
