As per Intent Market Research, the Mono Vaccines (Epstein-Barr Virus) Market was valued at USD 0.6 Billion in 2024-e and will surpass USD 1.1 Billion by 2030; growing at a CAGR of 10.7% during 2025-2030.
The Mono Vaccines (Epstein-Barr Virus) market is rapidly advancing, driven by increased awareness of the virus's link to various cancers and chronic illnesses. As research and development efforts intensify, innovative vaccine formulations are emerging to provide more effective prevention and treatment solutions. The rising prevalence of Epstein-Barr Virus-related diseases is catalyzing the growth of this market, creating opportunities for novel therapeutic and prophylactic solutions.
Therapeutic Vaccines Segment is Largest Owing to Comprehensive Disease Management
The Therapeutic Vaccines segment dominates the Mono Vaccines (Epstein-Barr Virus) market due to its ability to address the disease at a cellular level. These vaccines are designed to not only prevent the onset of EBV-related diseases but also to manage and mitigate symptoms in patients with existing conditions such as cancer and autoimmune disorders. The increasing focus on immune-boosting therapies and the demand for personalized medicine contribute to the dominance of therapeutic vaccines in this space.
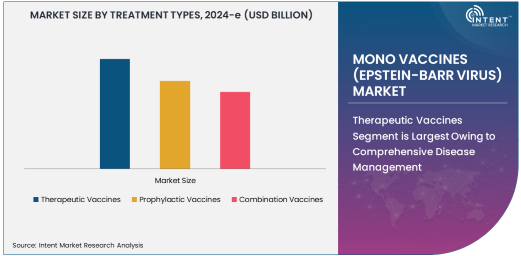
Prophylactic Vaccines Segment is Fastest Growing Owing to Prevention and Early Intervention
The Prophylactic Vaccines segment is the fastest-growing within the Mono Vaccines (Epstein-Barr Virus) market, driven by a surge in demand for preventative healthcare solutions. These vaccines are primarily aimed at healthy populations to prevent the initial infection and subsequent complications associated with EBV. With ongoing advancements in clinical research and rising public health initiatives, this segment is poised for rapid expansion in the coming years.
End-User Industries
The Pharmaceutical Companies segment is largest owing to their substantial investments in research, clinical trials, and the development of innovative vaccine formulations. These companies play a critical role in transforming scientific breakthroughs into market-ready products, fostering advancements in the treatment and prevention of Epstein-Barr Virus-related conditions.
Fastest Growing Region: Asia-Pacific
The Asia-Pacific region is the fastest-growing in the Mono Vaccines (Epstein-Barr Virus) market, driven by increasing healthcare infrastructure, rising prevalence of EBV-related diseases, and a growing demand for effective preventive solutions. Countries like China, India, and Japan are emerging as key contributors to the region's dynamic growth due to heightened awareness and government support for vaccine development and distribution.
Competitive Landscape
The competitive landscape of the Mono Vaccines (Epstein-Barr Virus) market features a mix of leading pharmaceutical giants and innovative biotech firms. Companies such as GSK, Pfizer, and Moderna are at the forefront, leveraging advanced research capabilities and strategic partnerships to develop cutting-edge vaccine technologies. Additionally, smaller biotech firms are rapidly gaining traction by focusing on specialized formulations and personalized vaccine solutions, further intensifying market competition.
Recent Developments:
- GSK launched a new Phase 3 clinical trial for its Epstein-Barr Virus vaccine candidate in early 2024.
- Pfizer announced the acquisition of a biotech firm specializing in EBV vaccine research in late 2023.
- Moderna received approval for a multi-million dollar investment in the development of an Epstein-Barr Virus vaccine technology.
- BioNTech partnered with a research institution to accelerate Epstein-Barr Virus vaccine trials.
- AbbVie completed the acquisition of a biotech company with promising EBV therapeutic vaccine candidates.
List of Leading Companies:
- GSK
- Pfizer
- Johnson & Johnson
- Moderna
- Novavax
- Sanofi
- BioNTech
- AstraZeneca
- CureVac
- Inovio Pharmaceuticals
- Regeneron Pharmaceuticals
- Merck & Co.
- Bharat Biotech
- BioFarma
- AbbVie
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 0.6 Billion |
|
Forecasted Value (2030) |
USD 1.1 Billion |
|
CAGR (2025 – 2030) |
10.7% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Mono Vaccines (Epstein-Barr Virus) Market By Treatment Types (Therapeutic Vaccines, Prophylactic Vaccines, Combination Vaccines), By End-User Industries (Hospitals, Research Institutions, Specialty Clinics, Pharmaceutical Companies), and By Region; Global Insights & Forecast (2023 – 2030) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
GSK, Pfizer, Johnson & Johnson, Moderna, Novavax, Sanofi, BioNTech, AstraZeneca, CureVac, Inovio Pharmaceuticals, Regeneron Pharmaceuticals, Merck & Co., Bharat Biotech, BioFarma, AbbVie |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Mono Vaccines (Epstein-Barr Virus) Market, by Treatment Types (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Therapeutic Vaccines |
|
4.2. Prophylactic Vaccines |
|
4.3. Combination Vaccines |
|
5. Mono Vaccines (Epstein-Barr Virus) Market, by End-User Industries (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Hospitals |
|
5.2. Research Institutions |
|
5.3. Specialty Clinics |
|
5.4. Pharmaceutical Companies |
|
6. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Regional Overview |
|
6.2. North America |
|
6.2.1. Regional Trends & Growth Drivers |
|
6.2.2. Barriers & Challenges |
|
6.2.3. Opportunities |
|
6.2.4. Factor Impact Analysis |
|
6.2.5. Technology Trends |
|
6.2.6. North America Mono Vaccines (Epstein-Barr Virus) Market, by Treatment Types |
|
6.2.7. North America Mono Vaccines (Epstein-Barr Virus) Market, by End-User Industries |
|
6.2.8. By Country |
|
6.2.8.1. US |
|
6.2.8.1.1. US Mono Vaccines (Epstein-Barr Virus) Market, by Treatment Types |
|
6.2.8.1.2. US Mono Vaccines (Epstein-Barr Virus) Market, by End-User Industries |
|
6.2.8.2. Canada |
|
6.2.8.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
6.3. Europe |
|
6.4. Asia-Pacific |
|
6.5. Latin America |
|
6.6. Middle East & Africa |
|
7. Competitive Landscape |
|
7.1. Overview of the Key Players |
|
7.2. Competitive Ecosystem |
|
7.2.1. Level of Fragmentation |
|
7.2.2. Market Consolidation |
|
7.2.3. Product Innovation |
|
7.3. Company Share Analysis |
|
7.4. Company Benchmarking Matrix |
|
7.4.1. Strategic Overview |
|
7.4.2. Product Innovations |
|
7.5. Start-up Ecosystem |
|
7.6. Strategic Competitive Insights/ Customer Imperatives |
|
7.7. ESG Matrix/ Sustainability Matrix |
|
7.8. Manufacturing Network |
|
7.8.1. Locations |
|
7.8.2. Supply Chain and Logistics |
|
7.8.3. Product Flexibility/Customization |
|
7.8.4. Digital Transformation and Connectivity |
|
7.8.5. Environmental and Regulatory Compliance |
|
7.9. Technology Readiness Level Matrix |
|
7.10. Technology Maturity Curve |
|
7.11. Buying Criteria |
|
8. Company Profiles |
|
8.1. GSK |
|
8.1.1. Company Overview |
|
8.1.2. Company Financials |
|
8.1.3. Product/Service Portfolio |
|
8.1.4. Recent Developments |
|
8.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
8.2. Pfizer |
|
8.3. Johnson & Johnson |
|
8.4. Moderna |
|
8.5. Novavax |
|
8.6. Sanofi |
|
8.7. BioNTech |
|
8.8. AstraZeneca |
|
8.9. CureVac |
|
8.10. Inovio Pharmaceuticals |
|
8.11. Regeneron Pharmaceuticals |
|
8.12. Merck & Co. |
|
8.13. Bharat Biotech |
|
8.14. BioFarma |
|
8.15. AbbVie |
|
9. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Mono Vaccines (Epstein-Barr Virus) Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Mono Vaccines (Epstein-Barr Virus) Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Mono Vaccines (Epstein-Barr Virus) Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
