As per Intent Market Research, the Microneedle Flu Vaccine Market was valued at USD 1.0 billion in 2024-e and will surpass USD 2.3 billion by 2030; growing at a CAGR of 15.4% during 2025 - 2030.
The microneedle flu vaccine market is rapidly gaining traction as a next-generation method for vaccine delivery, offering enhanced patient comfort and convenience compared to traditional needle-based injections. Microneedles, which are tiny, minimally invasive needles, are designed to painlessly penetrate the skin's outer layer, delivering vaccines effectively without the need for deeper injections. This innovative technology not only reduces discomfort but also enhances patient compliance and convenience, making it particularly appealing in pediatric and elderly populations who are often averse to traditional injections.
The demand for microneedle flu vaccines is rising due to the growing global need for more efficient and less invasive vaccination methods. Additionally, the COVID-19 pandemic has increased awareness and investment in alternative vaccine delivery technologies, further accelerating the development and adoption of microneedles for flu vaccination. As healthcare systems focus on improving vaccination rates, particularly in remote or underserved areas, the microneedle flu vaccine market is positioned for significant growth.
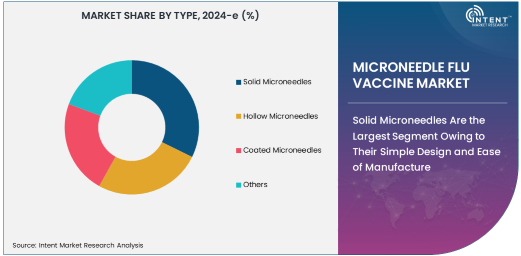
Solid Microneedles Are the Largest Segment Owing to Their Simple Design and Ease of Manufacture
Solid microneedles are the largest segment in the microneedle flu vaccine market, primarily due to their simple design and ease of manufacture. These microneedles consist of solid, needle-like structures that are designed to create micro-channels in the skin, allowing the vaccine to be absorbed without the need for deeper penetration. Their straightforward design and low production costs make them an attractive option for large-scale vaccine distribution.
The advantages of solid microneedles include ease of use, minimal risk of contamination, and the ability to be incorporated into pre-filled devices such as patches for easy self-administration. These benefits have driven widespread interest in solid microneedles for flu vaccines, especially as the demand for more accessible and user-friendly vaccination options grows. With ongoing improvements in their design and manufacturing processes, solid microneedles are expected to remain the leading technology in the microneedle flu vaccine market.
Intradermal Administration Route Is the Largest Segment Owing to Its Efficacy and Lower Pain Perception
The intradermal administration route is the largest segment in the microneedle flu vaccine market, owing to its efficacy and lower pain perception compared to traditional intramuscular injections. Intradermal injections involve delivering the vaccine into the skin layer, where immune cells are densely concentrated, leading to a strong immune response. The microneedles used for intradermal administration are small enough to avoid triggering the pain receptors in the deeper tissues, making this method a more comfortable option for patients.
Intradermal vaccination is particularly advantageous for flu vaccines as it requires a lower dose of the vaccine, reducing both cost and potential side effects. Additionally, the ability to self-administer vaccines via intradermal microneedles further enhances convenience and accessibility, driving the adoption of this administration route. As more studies confirm the effectiveness of intradermal microneedle delivery, it is expected to remain the preferred route for flu vaccination.
Patch-based Delivery Technology Is the Fastest Growing Owing to Its Ease of Use and Patient Compliance
Patch-based delivery technology is the fastest-growing segment in the microneedle flu vaccine market, primarily due to its ease of use and high patient compliance. Microneedle patches, which consist of arrays of tiny needles embedded on a patch, can be easily applied to the skin, delivering the vaccine without the need for specialized medical staff. This non-invasive method is particularly appealing for mass vaccination campaigns, as it allows individuals to self-administer the vaccine, reducing the strain on healthcare systems.
The growth of patch-based delivery is driven by its potential to simplify vaccine distribution, particularly in remote or underserved regions. As healthcare providers and governments increasingly focus on improving vaccination coverage, patch-based microneedles offer a cost-effective and scalable solution. The increasing demand for self-administration and the potential for integration into smart healthcare systems are expected to further accelerate the growth of this technology in the microneedle flu vaccine market.
Hospitals Are the Largest End-Use Industry Owing to Their High Patient Volume and Vaccine Distribution Infrastructure
Hospitals represent the largest end-use industry in the microneedle flu vaccine market, driven by their high patient volume and established vaccine distribution infrastructure. Hospitals are primary centers for flu vaccination campaigns, particularly during peak flu seasons, and are equipped with the necessary facilities for mass vaccination. As microneedle flu vaccines gain acceptance, hospitals are increasingly adopting these devices due to their ease of administration and the ability to handle large-scale vaccinations with reduced pain and patient anxiety.
The presence of trained medical staff and the capacity to provide comprehensive vaccination services make hospitals an ideal setting for the introduction of microneedle flu vaccines. As healthcare systems seek more efficient ways to manage patient flow and improve vaccination rates, hospitals will continue to dominate the microneedle flu vaccine market, ensuring the widespread adoption of this innovative technology.
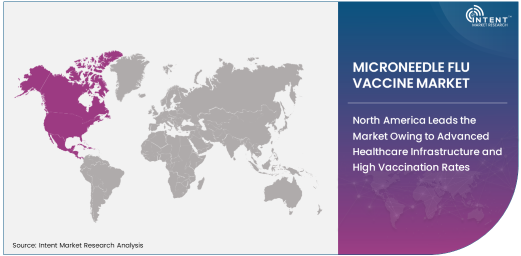
North America Leads the Market Owing to Advanced Healthcare Infrastructure and High Vaccination Rates
North America dominates the microneedle flu vaccine market, driven by the region's advanced healthcare infrastructure and high vaccination rates. The United States, in particular, has seen increased adoption of innovative vaccine delivery methods, including microneedle patches, due to their potential to offer painless, convenient, and effective vaccination solutions. The growing demand for non-invasive and self-administered vaccines among consumers further supports the market’s growth.
Additionally, government health programs and initiatives aimed at improving flu vaccination coverage and reducing needle-related concerns are accelerating the adoption of microneedle-based vaccines. The region's strong focus on research and development, along with the presence of leading biotech companies, positions North America as the market leader in microneedle flu vaccines.
Leading Companies and Competitive Landscape
The microneedle flu vaccine market is competitive, with several key players focusing on the development and commercialization of microneedle-based vaccine delivery technologies. Companies such as 3M, Micron Biomedical, and Vaxxas are at the forefront of this innovation, offering microneedle-based solutions that improve vaccine accessibility and patient comfort. These companies are investing heavily in research and development to enhance microneedle designs, improve manufacturing processes, and expand their product offerings across different vaccine types.
The competitive landscape is also characterized by collaborations between technology providers, pharmaceutical companies, and academic institutions, aimed at advancing microneedle vaccine technology and obtaining regulatory approvals. As the market evolves, companies that prioritize innovation, scalability, and patient-centric solutions will be well-positioned to lead the market. With continued advancements in microneedle technologies and their integration into broader healthcare systems, the microneedle flu vaccine market is set for sustained growth.
Recent Developments:
- In December 2024, Nanopass Technologies received funding for its microneedle-based flu vaccine project. The funding will help enhance the product's delivery mechanism and accelerate clinical trials.
- In November 2024, Vaxxas launched a new microneedle patch for flu vaccination. The patch is designed for easy, at-home use, offering a more accessible alternative to traditional vaccines.
- In October 2024, PharmaJet partnered with healthcare providers to introduce a needle-free flu vaccine delivery system. The collaboration aims to expand the accessibility of flu vaccines globally.
- In September 2024, Inovio Pharmaceuticals announced the successful preclinical results of its microneedle flu vaccine. The vaccine demonstrated significant efficacy and minimal side effects.
- In August 2024, Zosano Pharma expanded its microneedle technology to include flu vaccines. The company is targeting both commercial and developing markets with this innovation to improve vaccine distribution.
List of Leading Companies:
- Nanopass Technologies
- Vaxxas
- PharmaJet
- Micron Biomedical
- 3M
- Inovio Pharmaceuticals
- Zosano Pharma
- Dermarite Industries
- Vaxine Pty Ltd
- Bioject Medical Technologies
- MedImmune (AstraZeneca)
- Mitsubishi Tanabe Pharma
- Valeritas
- BD (Becton, Dickinson and Company)
- Microneedle Solutions
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 1.0 billion |
|
Forecasted Value (2030) |
USD 2.3 billion |
|
CAGR (2025 – 2030) |
15.4% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Microneedle Flu Vaccine Market By Type (Solid Microneedles, Hollow Microneedles, Coated Microneedles), By Administration Route (Intradermal, Transdermal), By Technology (Patch-based Delivery, Microneedle Array), By End-Use Industry (Hospitals, Clinics, Research & Development) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Nanopass Technologies, Vaxxas, PharmaJet, Micron Biomedical, 3M, Inovio Pharmaceuticals, Zosano Pharma, Dermarite Industries, Vaxine Pty Ltd, Bioject Medical Technologies, MedImmune (AstraZeneca), Mitsubishi Tanabe Pharma, Valeritas, BD (Becton, Dickinson and Company), Microneedle Solutions |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Microneedle Flu Vaccine Market, by Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Solid Microneedles |
|
4.2. Hollow Microneedles |
|
4.3. Coated Microneedles |
|
4.4. Others |
|
5. Microneedle Flu Vaccine Market, by Administration Route (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Intradermal |
|
5.2. Transdermal |
|
5.3. Others |
|
6. Microneedle Flu Vaccine Market, by Technology (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Patch-based Delivery |
|
6.2. Microneedle Array |
|
6.3. Others |
|
7. Microneedle Flu Vaccine Market, by End-Use Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Hospitals |
|
7.2. Clinics |
|
7.3. Research & Development |
|
7.4. Others |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America Microneedle Flu Vaccine Market, by Type |
|
8.2.7. North America Microneedle Flu Vaccine Market, by Administration Route |
|
8.2.8. North America Microneedle Flu Vaccine Market, by Technology |
|
8.2.9. North America Microneedle Flu Vaccine Market, by End-Use Industry |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US Microneedle Flu Vaccine Market, by Type |
|
8.2.10.1.2. US Microneedle Flu Vaccine Market, by Administration Route |
|
8.2.10.1.3. US Microneedle Flu Vaccine Market, by Technology |
|
8.2.10.1.4. US Microneedle Flu Vaccine Market, by End-Use Industry |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Nanopass Technologies |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Vaxxas |
|
10.3. PharmaJet |
|
10.4. Micron Biomedical |
|
10.5. 3M |
|
10.6. Inovio Pharmaceuticals |
|
10.7. Zosano Pharma |
|
10.8. Dermarite Industries |
|
10.9. Vaxine Pty Ltd |
|
10.10. Bioject Medical Technologies |
|
10.11. MedImmune (AstraZeneca) |
|
10.12. Mitsubishi Tanabe Pharma |
|
10.13. Valeritas |
|
10.14. BD (Becton, Dickinson and Company) |
|
10.15. Microneedle Solutions |
|
11. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Microneedle Flu Vaccine Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Microneedle Flu Vaccine Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Microneedle Flu Vaccine Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
