As per Intent Market Research, the Medical Robotic Systems Market was valued at USD 28.2 Billion in 2024-e and will surpass USD 72.2 Billion by 2030; growing at a CAGR of 17.0% during 2025-2030.
The Medical Robotic Systems market is experiencing significant growth, driven by advancements in technology and an increasing demand for precision, minimally invasive surgeries, and rehabilitation solutions. Robotic systems are transforming the healthcare landscape by enhancing surgical accuracy, reducing recovery times, and improving patient outcomes. These systems are widely used in various medical applications, including robotic surgeries, prosthetics, and rehabilitation therapies.
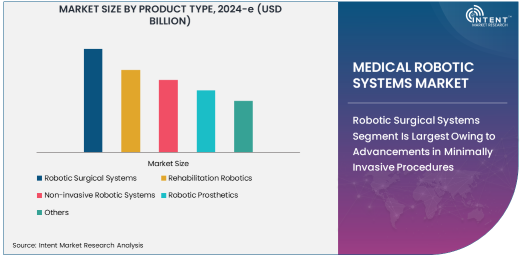
Robotic Surgical Systems Segment Is Largest Owing to Advancements in Minimally Invasive Procedures
The medical robotic systems market has seen exponential growth, driven by technological advancements and rising demand for minimally invasive procedures. Among the various product types, robotic surgical systems have emerged as the largest segment. These systems are widely used in surgeries that require high precision and minimal incisions, such as those in orthopedics, urology, and gynecology. Robotic surgical systems like the da Vinci Surgical System have revolutionized surgeries by enabling surgeons to perform complex procedures with enhanced precision, flexibility, and control. As a result, these systems are becoming increasingly popular in hospitals and surgical centers worldwide.
The adoption of robotic surgical systems is largely driven by their ability to reduce patient recovery time and improve surgical outcomes. Hospitals, particularly in developed regions, have been at the forefront of adopting these systems to offer patients advanced surgical options. This segment's growth is also supported by continuous innovation and the increasing affordability of robotic systems, which have made them more accessible to a wider range of healthcare providers.
Minimally Invasive Surgery Application Is Fastest Growing Owing to Rising Demand for Faster Recovery
In the medical robotic systems market, minimally invasive surgery (MIS) is the fastest-growing application segment. MIS allows for the performance of complex surgeries with small incisions, which significantly reduces recovery time, the risk of infection, and overall patient discomfort. The increasing preference for procedures that are less invasive, coupled with the benefits of faster recovery and lower healthcare costs, has led to a surge in demand for robotic systems used in MIS. This trend is particularly evident in fields such as urology, gynecology, and colorectal surgery, where robotic systems have demonstrated superior precision and operational efficiency.
The MIS segment's growth is driven by technological advancements in robotic systems that enable more complex and delicate procedures to be performed with greater precision. Surgeons can control robotic arms with enhanced dexterity, which is crucial for performing minimally invasive procedures with higher success rates. As patient preferences lean toward shorter hospital stays and reduced recovery times, the demand for robotic systems in MIS applications is expected to increase, further accelerating the growth of this segment.
Hospitals Segment Is Largest End-User Owing to High Adoption of Robotic Surgery Systems
Among the various end-users in the medical robotic systems market, hospitals represent the largest segment. The adoption of robotic surgical systems in hospitals is driven by their ability to enhance surgical outcomes and improve patient recovery times. Hospitals, especially in developed markets such as North America and Europe, are increasingly investing in advanced robotic systems to offer minimally invasive surgical options. These systems improve operational efficiency and enable surgeons to perform highly complex procedures with greater precision, flexibility, and control.
Hospitals continue to lead the market for robotic systems owing to their large-scale infrastructure, which allows them to integrate robotic systems into their surgical departments. The growing trend of specialized hospitals offering cutting-edge medical procedures has further contributed to the dominance of hospitals in the end-user segment. As more hospitals adopt robotic surgery systems, the demand for these technologies is expected to rise, driving the overall growth of the medical robotic systems market.
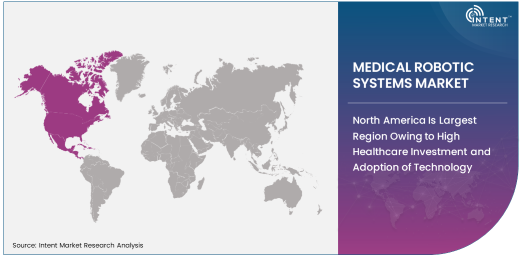
North America Is Largest Region Owing to High Healthcare Investment and Adoption of Technology
North America remains the largest region in the medical robotic systems market. The region is home to some of the most advanced healthcare systems in the world, with significant investments in research and development, healthcare infrastructure, and the adoption of advanced medical technologies. The presence of leading companies such as Intuitive Surgical, Medtronic, and Stryker, along with favorable reimbursement policies, has propelled the adoption of robotic systems in surgeries across hospitals and ambulatory surgery centers. Additionally, North America has a high number of surgical procedures, including those for cardiac, orthopedic, and neurological conditions, which directly drives the demand for robotic systems in these fields.
Furthermore, the increasing demand for minimally invasive surgeries in North America, coupled with a high level of awareness about the benefits of robotic surgery, is contributing to the region's dominance in the market. The rising incidence of chronic diseases and an aging population are expected to further fuel the demand for robotic surgical systems in the coming years, solidifying North America's position as the largest regional market.
Leading Companies and Competitive Landscape
The medical robotic systems market is highly competitive, with several prominent players leading the charge. Intuitive Surgical, known for its da Vinci Surgical System, is a dominant force in the market, with a strong market share and extensive product offerings in robotic surgery. Other major players include Medtronic, which offers robotic-assisted surgery platforms like Hugo™, and Stryker, with its Mako™ robotic system for orthopedic surgeries. Johnson & Johnson and Zimmer Biomet are also key players, focusing on expanding their robotic surgical systems for orthopedic and joint replacement surgeries.
The competitive landscape is marked by continuous innovation, with companies investing heavily in R&D to develop next-generation robotic systems. Partnerships, acquisitions, and mergers are common strategies used by market leaders to gain a competitive edge and expand their product portfolios. For instance, Medtronic’s acquisition of Mazor Robotics strengthened its position in the robotic spinal surgery market. With ongoing technological advancements and increasing adoption of robotic systems in hospitals and surgery centers, the competitive dynamics in the market will continue to evolve.
Recent Developments:
- Medtronic PLC announced the launch of its Hugo™ robotic-assisted surgery platform upgrade, featuring enhanced capabilities for minimally invasive procedures, allowing improved precision.
- Intuitive Surgical Inc. expanded its da Vinci™ surgical system by introducing a new software update to enhance visualization and precision in robotic surgeries.
- Zimmer Biomet secured FDA approval for its ROSATM robotic system, designed for orthopedic surgeries to improve the accuracy and efficiency of joint replacements.
- Johnson & Johnson acquired Orthotaxy, a company focused on developing robotic-assisted orthopedic surgery solutions to enhance its robotic surgery offerings.
- Stryker Corporation announced its acquisition of Mako Surgical Corp., a leader in robotic arm-assisted surgery, enhancing its capabilities in orthopedic procedures.
List of Leading Companies:
- Intuitive Surgical Inc.
- Medtronic PLC
- Johnson & Johnson
- Stryker Corporation
- Zimmer Biomet
- Siemens Healthineers AG
- Smith & Nephew PLC
- Accuray Incorporated
- Mako Surgical Corp.
- Corindus Vascular Robotics
- TransEnterix Inc.
- Aesculap Inc.
- CMR Surgical
- Brainlab AG
- KUKA Robotics Corporation
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 28.2 Billion |
|
Forecasted Value (2030) |
USD 72.2 Billion |
|
CAGR (2025 – 2030) |
17.0% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Medical Robotic Systems Market By Product Type (Robotic Surgical Systems, Rehabilitation Robotics, Non-invasive Robotic Systems, Robotic Prosthetics), By Application (Minimally Invasive Surgery, Orthopedic Surgery, Neurosurgery, Cardiac Surgery, Urology, Gynecology, Rehabilitation), By End-User (Hospitals, Ambulatory Surgery Centers, Research Institutes, Rehabilitation Centers) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Intuitive Surgical Inc., Medtronic PLC, Johnson & Johnson, Stryker Corporation, Zimmer Biomet, Siemens Healthineers AG, Smith & Nephew PLC, Accuray Incorporated, Mako Surgical Corp., Corindus Vascular Robotics, TransEnterix Inc., Aesculap Inc., CMR Surgical, Brainlab AG, KUKA Robotics Corporation |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Medical Robotic Systems Market, by By Product Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Robotic Surgical Systems |
|
4.2. Rehabilitation Robotics |
|
4.3. Non-invasive Robotic Systems |
|
4.4. Robotic Prosthetics |
|
4.5. Others |
|
5. Medical Robotic Systems Market, by Application (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Minimally Invasive Surgery |
|
5.2. Orthopedic Surgery |
|
5.3. Neurosurgery |
|
5.4. Cardiac Surgery |
|
5.5. Urology |
|
5.6. Gynecology |
|
5.7. Rehabilitation |
|
5.8. Others |
|
6. Medical Robotic Systems Market, by End-User (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Hospitals |
|
6.2. Ambulatory Surgery Centers |
|
6.3. Research Institutes |
|
6.4. Rehabilitation Centers |
|
6.5. Others |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Medical Robotic Systems Market, by By Product Type |
|
7.2.7. North America Medical Robotic Systems Market, by Application |
|
7.2.8. North America Medical Robotic Systems Market, by End-User |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Medical Robotic Systems Market, by By Product Type |
|
7.2.9.1.2. US Medical Robotic Systems Market, by Application |
|
7.2.9.1.3. US Medical Robotic Systems Market, by End-User |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. Intuitive Surgical Inc. |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. Medtronic PLC |
|
9.3. Johnson & Johnson |
|
9.4. Stryker Corporation |
|
9.5. Zimmer Biomet |
|
9.6. Siemens Healthineers AG |
|
9.7. Smith & Nephew PLC |
|
9.8. Accuray Incorporated |
|
9.9. Mako Surgical Corp. |
|
9.10. Corindus Vascular Robotics |
|
9.11. TransEnterix Inc. |
|
9.12. Aesculap Inc. |
|
9.13. CMR Surgical |
|
9.14. Brainlab AG |
|
9.15. KUKA Robotics Corporation |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Medical Robotic Systems Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Medical Robotic Systems Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Medical Robotic Systems Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
