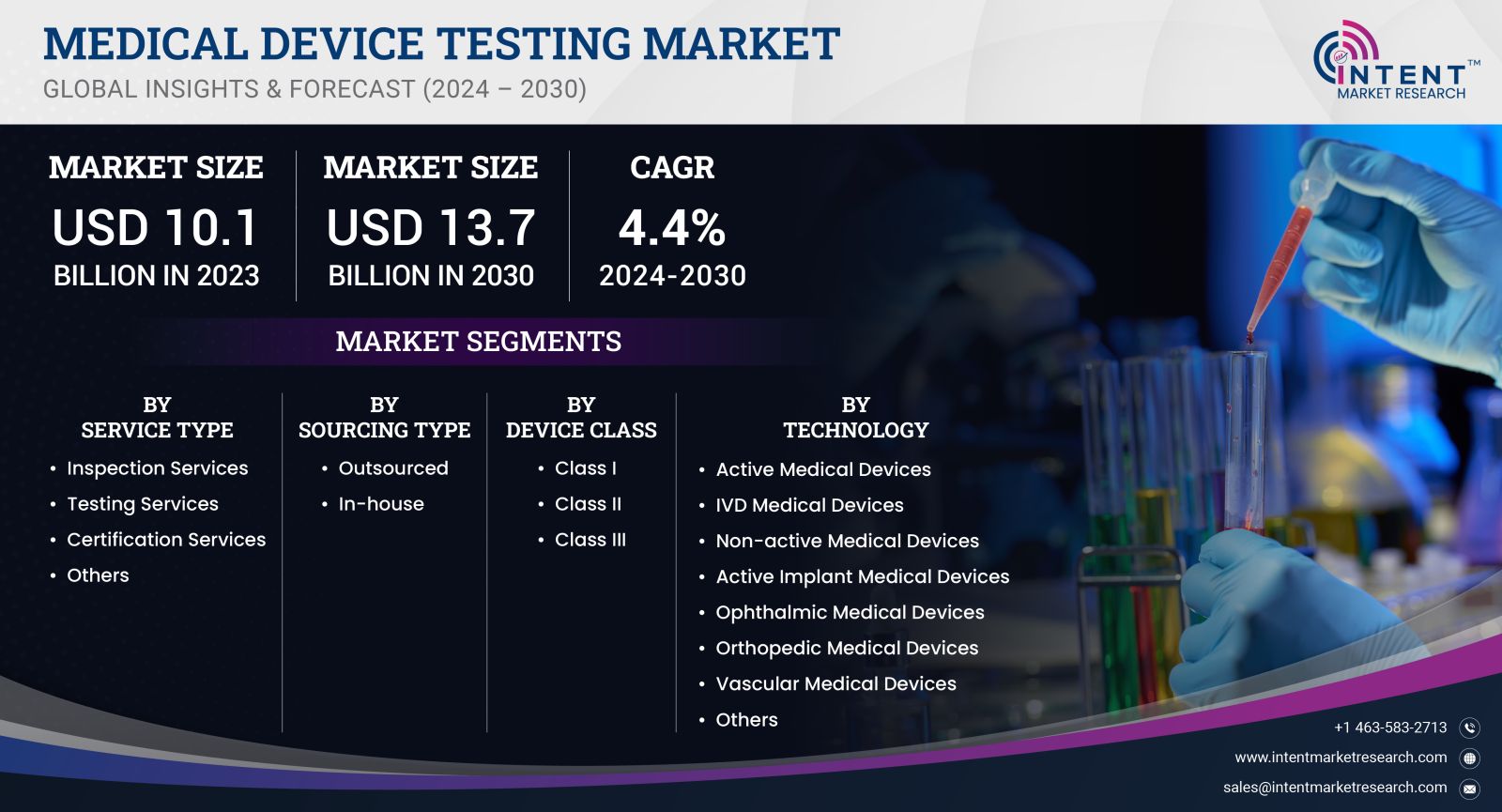
As per Intent Market Research, the Medical Device Testing Market was valued at USD 10.1 billion in 2023-e and will surpass USD 13.7 billion by 2030; growing at a CAGR of 4.4% during 2024 - 2030.
The medical device testing market is witnessing significant growth due to the increasing complexity of medical devices and the rising emphasis on safety and efficacy in healthcare. Medical devices, ranging from simple instruments to sophisticated software applications, require rigorous testing to ensure they meet regulatory standards and perform effectively in real-world applications.
Key factors driving growth in the medical device testing market include the advancement of technology, the rise in regulatory requirements, and the growing demand for innovative healthcare solutions. As medical devices become more integrated with digital technologies, the testing landscape is evolving to encompass a wider range of evaluations, including cybersecurity and software validation. The increasing adoption of connected medical devices and the focus on patient safety further underscore the importance of thorough testing and validation processes.
In Vitro Diagnostic (IVD) Testing Segment is Largest Owing to Rising Disease Incidences
The in vitro diagnostic (IVD) testing segment is the largest within the medical device testing market, driven by the rising incidence of chronic diseases and the growing demand for accurate diagnostic tools. IVD tests, which analyze samples such as blood, urine, and tissue to provide critical health information, are essential for early disease detection and management. The increasing prevalence of conditions such as diabetes, cancer, and infectious diseases is fueling the demand for advanced IVD testing solutions.
Furthermore, technological advancements in diagnostic testing, including the development of point-of-care testing devices, are enhancing the accessibility and efficiency of healthcare delivery. These innovations are enabling healthcare professionals to make timely decisions based on rapid test results, thereby improving patient outcomes. As a result, the IVD testing segment is expected to maintain its dominance in the medical device testing market, with a projected CAGR of approximately 7.5% from 2024 to 2030.
Quality Assurance and Regulatory Compliance Segment is Fastest Growing Owing to Stringent Regulations
The quality assurance and regulatory compliance segment is the fastest-growing segment in the medical device testing market, driven by the increasing stringency of regulatory requirements imposed by agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Medical device manufacturers are under immense pressure to ensure that their products meet stringent safety and quality standards before they can be marketed. This has led to a growing emphasis on comprehensive testing protocols and documentation practices.
Additionally, as the global medical device market expands, the need for consistent quality assurance across different regions is becoming paramount. Manufacturers are increasingly seeking testing services that can provide guidance through the complex regulatory landscape, ensuring compliance with local and international standards. With a projected CAGR of around 8% from 2024 to 2030, the quality assurance and regulatory compliance segment is poised for significant growth, reflecting the heightened focus on safety and efficacy in medical device development.
Biomedical Testing Segment is Largest Owing to Technological Advancements
The biomedical testing segment is the largest in the medical device testing market, primarily due to advancements in technology that enhance the reliability and accuracy of testing processes. Biomedical testing encompasses a broad range of services, including biocompatibility testing, sterilization validation, and materials testing. These services are essential for ensuring that medical devices are safe for human use and meet the necessary regulatory standards.
Moreover, the increasing complexity of medical devices necessitates comprehensive biomedical testing to identify potential risks associated with new materials and technologies. The growing demand for innovative and customized medical devices further drives the need for specialized testing services that can adapt to evolving industry standards. As a result, the biomedical testing segment is expected to continue its significant growth trajectory, with an anticipated CAGR of approximately 6.5% from 2024 to 2030.
Fastest Growing Region is Asia-Pacific Owing to Rising Healthcare Investments
The Asia-Pacific region is the fastest-growing market for medical device testing, driven by increasing investments in healthcare infrastructure and the rising demand for advanced medical technologies. Countries such as China, India, and Japan are experiencing rapid growth in their healthcare sectors, leading to a heightened need for comprehensive testing services to support the development of innovative medical devices. The region's growing population and increasing prevalence of chronic diseases further underscore the importance of reliable medical device testing.
Additionally, the push for regulatory harmonization and the adoption of international standards in Asia-Pacific countries are encouraging local manufacturers to invest in testing services that ensure compliance with global market requirements. As the region continues to develop its healthcare capabilities, the medical device testing market in Asia-Pacific is projected to grow at a remarkable CAGR of around 9% from 2024 to 2030, positioning it as a key player in the global landscape.
Competitive Landscape and Leading Companies
The competitive landscape of the medical device testing market is characterized by the presence of numerous established companies and emerging players that are actively investing in innovation and expanding their service offerings. Leading companies in this sector include TÜV SÜD AG, Intertek Group plc, SGS SA, BSI Group, UL LLC, Eurofins Scientific SE, Nelson Labs LLC, BioTek Instruments, Inc., NAMSA, and MDSAP. These organizations are recognized for their expertise in providing comprehensive testing solutions that meet the diverse needs of the medical device industry.
Collaboration and partnerships are common strategies among these market leaders, enabling them to leverage complementary technologies and expertise to enhance their testing capabilities. Additionally, mergers and acquisitions are reshaping the competitive landscape, allowing companies to broaden their market presence and expand their service portfolios. As the medical device testing market continues to evolve, these leading players are well-positioned to capitalize on emerging opportunities, driving innovation and growth in this vital industry. With a focus on meeting regulatory requirements and ensuring product safety, the competitive landscape is set for further evolution in the coming years.
Report Objectives:
The report will help you answer some of the most critical questions in the Medical Device Testing Market. A few of them are as follows:
- What are the key drivers, restraints, opportunities, and challenges influencing the market growth?
- What are the prevailing technology trends in the Medical Device Testing market?
- What is the size of the Medical Device Testing market based on segments, sub-segments, and regions?
- What is the size of different market segments across key regions: North America, Europe, Asia Pacific, Latin America, Middle East & Africa?
- What are the market opportunities for stakeholders after analyzing key market trends?
- Who are the leading market players and what are their market share and core competencies?
- What is the degree of competition in the market and what are the key growth strategies adopted by leading players?
- What is the competitive landscape of the market, including market share analysis, revenue analysis, and a ranking of key players?
Report Scope:
|
Report Features |
Description |
|
Market Size (2023-e) |
USD 10.1 billion |
|
Forecasted Value (2030) |
USD 13.7 billion |
|
CAGR (2024-2030) |
4.4% |
|
Base Year for Estimation |
2023-e |
|
Historic Year |
2022 |
|
Forecast Period |
2024-2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Medical Device Testing Market By Service (Inspection, Testing, Certification), By Sourcing (Outsourced, In-house), By Device Class (I, II, III), By Technology (Active Medical Devices, Active Implant, Non-active Medical Devices, IVD) |
|
Regional Analysis |
North America (US, Canada), Europe (Germany, France, UK, Spain, Italy & Rest of Europe), Asia Pacific (China, Japan, South Korea, India, and rest of Asia Pacific), Latin America (Brazil, Mexico, Argentina, & Rest of Latin America), Middle East & Africa (Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA) |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1.Introduction |
|
1.1.Market Definition |
|
1.2.Scope of the Study |
|
1.3.Research Assumptions |
|
1.4.Study Limitations |
|
2.Research Methodology |
|
2.1.Research Approach |
|
2.1.1.Top-Down Method |
|
2.1.2.Bottom-Up Method |
|
2.1.3.Factor Impact Analysis |
|
2.2.Insights & Data Collection Process |
|
2.2.1.Secondary Research |
|
2.2.2.Primary Research |
|
2.3.Data Mining Process |
|
2.3.1.Data Analysis |
|
2.3.2.Data Validation and Revalidation |
|
2.3.3.Data Triangulation |
|
3.Executive Summary |
|
3.1.Major Markets & Segments |
|
3.2.Highest Growing Regions and Respective Countries |
|
3.3.Impact of Growth Drivers & Inhibitors |
|
3.4.Regulatory Overview by Country |
|
4.Medical Device Testing Market, by Service Type (Market Size & Forecast: USD Billion, 2024 – 2030) |
|
4.1.Inspection Services |
|
4.2.Testing Services |
|
4.3.Certification Services |
|
4.4.Others |
|
5.Medical Device Testing Market, by Sourcing Type (Market Size & Forecast: USD Billion, 2024 – 2030) |
|
5.1.Outsourced |
|
5.2.In-house |
|
6.Medical Device Testing Market, by Device Class (Market Size & Forecast: USD Billion, 2024 – 2030) |
|
6.1.Class I |
|
6.2.Class II |
|
6.3.Class III |
|
7.Medical Device Testing Market, by Technology (Market Size & Forecast: USD Billion, 2024 – 2030) |
|
7.1.Active Medical Devices |
|
7.2.IVD Medical Devices |
|
7.3.Non-active Medical Devices |
|
7.4.Active Implant Medical Devices |
|
7.5.Ophthalmic Medical Devices |
|
7.6.Orthopedic Medical Devices |
|
7.7.Vascular Medical Devices |
|
7.8.Others |
|
8.Regional Analysis (Market Size & Forecast: USD Billion, 2024 – 2030) |
|
8.1.Regional Overview |
|
8.2.North America |
|
8.2.1.Regional Trends & Growth Drivers |
|
8.2.2.Barriers & Challenges |
|
8.2.3.Opportunities |
|
8.2.4.Factor Impact Analysis |
|
8.2.5.Technology Trends |
|
8.2.6.North America Medical Device Testing Market, by Service Type |
|
8.2.7.North America Medical Device Testing Market, by Sourcing Type |
|
8.2.8.North America Medical Device Testing Market, by Device Class |
|
8.2.9.North America Medical Device Testing Market, by Technology |
|
*Similar segmentation will be provided at each regional level |
|
8.3.By Country |
|
8.3.1.US |
|
8.3.1.1.US Medical Device Testing Market, by Service Type |
|
8.3.1.2.US Medical Device Testing Market, by Sourcing Type |
|
8.3.1.3.US Medical Device Testing Market, by Device Class |
|
8.3.1.4.US Medical Device Testing Market, by Technology |
|
8.3.2.Canada |
|
*Similar segmentation will be provided at each country level |
|
8.4.Europe |
|
8.5.APAC |
|
8.6.Latin America |
|
8.7.Middle East & Africa |
|
9.Competitive Landscape |
|
9.1.Overview of the Key Players |
|
9.2.Competitive Ecosystem |
|
9.2.1.Platform Manufacturers |
|
9.2.2.Subsystem Manufacturers |
|
9.2.3.Service Providers |
|
9.2.4.Software Providers |
|
9.3.Company Share Analysis |
|
9.4.Company Benchmarking Matrix |
|
9.4.1.Strategic Overview |
|
9.4.2.Product Innovations |
|
9.5.Start-up Ecosystem |
|
9.6.Strategic Competitive Insights/ Customer Imperatives |
|
9.7.ESG Matrix/ Sustainability Matrix |
|
9.8.Manufacturing Network |
|
9.8.1.Locations |
|
9.8.2.Supply Chain and Logistics |
|
9.8.3.Product Flexibility/Customization |
|
9.8.4.Digital Transformation and Connectivity |
|
9.8.5.Environmental and Regulatory Compliance |
|
9.9.Technology Readiness Level Matrix |
|
9.10.Technology Maturity Curve |
|
9.11.Buying Criteria |
|
10.Company Profiles |
|
10.1.Sotera Health |
|
10.1.1.Company Overview |
|
10.1.2.Company Financials |
|
10.1.3.Product/Service Portfolio |
|
10.1.4.Recent Developments |
|
10.1.5.IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2.Intertek Group |
|
10.3.SGS |
|
10.4.Bureau Veritas |
|
10.5.TÜV Rheinland |
|
10.6.Eurofins Scientific |
|
10.7.TÜV SUD |
|
10.8.Charles River Laboratories |
|
10.9.Laboratory Corporation Of America |
|
10.10.Inotiv |
|
11.Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Medical Device Testing Market. In the process, the analysis was also done to estimate the parent market and relevant adjacencies to major the impact of them on the Medical Device Testing Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the Medical Device Testing ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Estimation
A combination of top-down and bottom-up approaches was utilized to estimate the overall size of the Medical Device Testing market. These methods were also employed to estimate the size of various sub-segments within the market. The market size estimation methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size estimates, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size estimates.
NA