As per Intent Market Research, the Lysosomal Storage Diseases Therapeutics Market was valued at USD 9.7 Billion in 2024-e and will surpass USD 16.9 Billion by 2030; growing at a CAGR of 9.8% during 2025-2030.
The global lysosomal storage diseases (LSDs) therapeutics market is driven by the rising prevalence of rare genetic disorders and the increasing availability of advanced treatments. LSDs represent a group of inherited metabolic disorders caused by the dysfunction of lysosomal enzymes, leading to the accumulation of substrates within cells. These diseases often result in severe health complications and can be life-threatening. Over the years, treatment modalities have evolved, with enzyme replacement therapy (ERT) being the primary approach for managing many LSDs. Other treatments, such as gene therapy and stem cell transplantation, are gaining momentum as potential cures. With research and development efforts intensifying, the market for LSD therapeutics is projected to grow at a steady pace in the coming years, with certain regions leading the charge in terms of innovation and patient access.
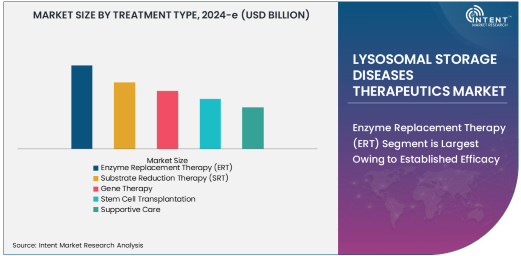
Enzyme Replacement Therapy (ERT) Segment is Largest Owing to Established Efficacy
Enzyme replacement therapy (ERT) remains the largest segment in the lysosomal storage diseases therapeutics market due to its proven efficacy and widespread use. ERT involves the intravenous infusion of synthetic enzymes to replace the deficient or absent enzymes in patients with LSDs, such as Gaucher disease, Fabry disease, and Pompe disease. It has been extensively studied and has shown significant positive clinical outcomes in improving symptoms, preventing disease progression, and enhancing the quality of life for patients. Major pharmaceutical companies such as Sanofi and Pfizer have already established a strong foothold in this segment, making it the dominant treatment modality.
The continued advancements in ERT formulations, along with the development of more patient-friendly delivery mechanisms, are expected to further bolster its position in the LSDs therapeutic landscape. With regulatory approvals for various ERT products already in place, and ongoing trials for new indications, the future for this treatment type remains promising.
Pompe Disease is Fastest Growing Disease Type Due to Increased Awareness and Treatment Advances
Pompe disease is the fastest growing disease type in the LSDs therapeutics market due to increasing awareness, improved diagnosis, and advancements in treatment options. Pompe disease is a rare, inherited metabolic disorder caused by the deficiency of the enzyme acid alpha-glucosidase (GAA), which leads to muscle weakness and respiratory failure. With more targeted therapies and enzyme replacement options now available, the disease is becoming more manageable. The introduction of innovative ERTs has contributed significantly to improving the quality of life of patients with Pompe disease, driving greater demand for specialized therapeutics.
As healthcare systems improve their recognition and diagnosis of Pompe disease, the patient pool for treatment is expanding. Ongoing research into gene therapy and other novel treatments is expected to further accelerate the growth of the Pompe disease segment, establishing it as a key area of focus in the LSDs therapeutic market.
Intravenous Route of Administration is Most Commonly Used Due to Effectiveness in Delivery
The intravenous (IV) route of administration remains the most common and effective method for delivering treatment to patients with LSDs, especially for Enzyme Replacement Therapy (ERT). The IV administration allows for more precise and controlled delivery of therapeutic enzymes directly into the bloodstream, which is crucial for patients who require long-term, consistent treatment. This method ensures higher bioavailability and quicker therapeutic effects, making it the preferred choice for many LSD treatments.
Given the critical nature of the diseases and the need for precise dosing, IV treatments offer patients the best option for disease management. As a result, the intravenous segment continues to dominate the LSDs therapeutics market and is expected to maintain its leadership position as more advanced treatments become available.
Hospitals are the Largest End-User Segment for LSDs Treatments
Hospitals remain the largest end-user segment for LSDs treatments, primarily due to the high level of care required for managing these complex disorders. Hospitals offer comprehensive services, including specialized diagnostics, enzyme replacement therapy administration, and continuous monitoring of patients with LSDs. Patients undergoing long-term treatments such as intravenous Enzyme Replacement Therapy (ERT) often require hospital-based care for regular infusions, making hospitals the key setting for treatment delivery.
Moreover, hospitals are typically equipped with the necessary infrastructure and medical expertise to handle the complexities of LSDs, making them the preferred choice for both patients and healthcare providers. As new treatment modalities, including gene therapy, become more widely available, hospitals will continue to play a central role in ensuring the effective delivery of these therapies.
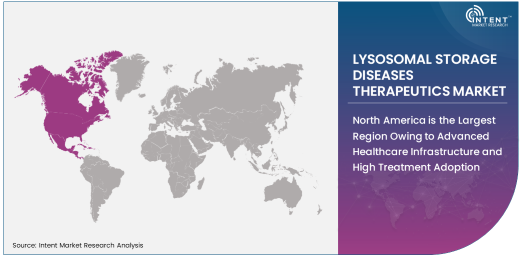
North America is the Largest Region Owing to Advanced Healthcare Infrastructure and High Treatment Adoption
North America leads the LSDs therapeutics market, driven by its advanced healthcare infrastructure, high treatment adoption rates, and strong regulatory frameworks. The United States, in particular, has a robust healthcare system that offers widespread access to the latest treatments for rare diseases, including LSDs. The presence of major pharmaceutical companies and research institutions in North America further contributes to the region's dominance in the market. Additionally, the high prevalence of LSDs in the region and increasing patient awareness have spurred demand for effective therapies.
North America's large market share is also supported by government initiatives and healthcare policies that provide financial support for the treatment of rare diseases. As the region continues to innovate in gene therapies and enzyme replacement treatments, it is expected to maintain its leadership in the global LSDs therapeutics market.
Competitive Landscape and Leading Companies
The lysosomal storage diseases therapeutics market is characterized by strong competition, with several leading pharmaceutical companies actively developing and commercializing treatments. Companies such as Sanofi, Pfizer, and BioMarin Pharmaceutical have established themselves as key players in the market with their Enzyme Replacement Therapy (ERT) products. These companies are also investing heavily in research and development to expand their product portfolios and improve treatment outcomes for patients with LSDs.
In addition to ERT, gene therapy and substrate reduction therapy (SRT) are gaining traction as promising treatment options, with companies like Amicus Therapeutics, Alexion Pharmaceuticals, and Ultragenyx Pharmaceutical leading the way in these innovative approaches. The competitive landscape is dynamic, with numerous ongoing clinical trials and a strong pipeline of therapies expected to shape the future of LSDs treatments. Collaboration and strategic partnerships are also key trends in the market, as companies seek to accelerate the development and commercialization of new therapies to address the unmet needs of patients with rare diseases.
Recent Developments:
- Pfizer completed its acquisition of Biohaven to strengthen its portfolio in neuroscience, including lyophilized products for migraine treatment.
- Sanofi announced a $500 million investment in its vaccine manufacturing plant, incorporating advanced lyophilization capabilities.
- Merck launched a new monoclonal antibody product using advanced lyophilization techniques to enhance stability and efficacy.
- AstraZeneca announced a strategic partnership to streamline its lyophilization process for its oncology portfolio.
- The FDA approved GSK's lyophilized vaccine for respiratory diseases, reinforcing its focus on innovative freeze-dried products.
List of Leading Companies:
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- BioMarin Pharmaceutical Inc.
- Pfizer Inc.
- Amicus Therapeutics, Inc.
- Alexion Pharmaceuticals, Inc.
- Ultragenyx Pharmaceutical Inc.
- Orchard Therapeutics plc
- Freeline Therapeutics
- Spark Therapeutics, Inc.
- JCR Pharmaceuticals Co., Ltd.
- Eli Lilly and Company
- Protalix BioTherapeutics, Inc.
- Horizon Therapeutics plc
- Sangamo Therapeutics, Inc.
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 9.7 Billion |
|
Forecasted Value (2030) |
USD 16.9 Billion |
|
CAGR (2025 – 2030) |
9.8% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Lysosomal Storage Diseases Therapeutics Market By Treatment Type (Enzyme Replacement Therapy, Substrate Reduction Therapy, Gene Therapy, Stem Cell Transplantation, Supportive Care), By Disease Type (Gaucher Disease, Fabry Disease, Pompe Disease, Mucopolysaccharidosis Disorders, Niemann-Pick Disease), By Route of Administration (Intravenous, Oral), By End-User (Hospitals, Specialty Clinics, Research Institutes, Homecare Settings) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Sanofi S.A., Takeda Pharmaceutical Company Limited, BioMarin Pharmaceutical Inc., Pfizer Inc., Amicus Therapeutics, Inc., Alexion Pharmaceuticals, Inc., Ultragenyx Pharmaceutical Inc., Orchard Therapeutics plc, Freeline Therapeutics, Spark Therapeutics, Inc., JCR Pharmaceuticals Co., Ltd., Eli Lilly and Company, Protalix BioTherapeutics, Inc., Horizon Therapeutics plc, Sangamo Therapeutics, Inc. |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Lysosomal Storage Diseases Therapeutics Market, by Treatment Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Enzyme Replacement Therapy (ERT) |
|
4.2. Substrate Reduction Therapy (SRT) |
|
4.3. Gene Therapy |
|
4.4. Stem Cell Transplantation |
|
4.5. Supportive Care |
|
5. Lysosomal Storage Diseases Therapeutics Market, by Disease Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Gaucher Disease |
|
5.2. Fabry Disease |
|
5.3. Pompe Disease |
|
5.4. Mucopolysaccharidosis (MPS) Disorders |
|
5.5. Niemann-Pick Disease |
|
5.6. Others |
|
6. Lysosomal Storage Diseases Therapeutics Market, by Route of Administration (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Intravenous |
|
6.2. Oral |
|
7. Lysosomal Storage Diseases Therapeutics Market, by End-User (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Hospitals |
|
7.2. Specialty Clinics |
|
7.3. Research Institutes |
|
7.4. Homecare Settings |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America Lysosomal Storage Diseases Therapeutics Market, by Treatment Type |
|
8.2.7. North America Lysosomal Storage Diseases Therapeutics Market, by Disease Type |
|
8.2.8. North America Lysosomal Storage Diseases Therapeutics Market, by Route of Administration |
|
8.2.9. North America Lysosomal Storage Diseases Therapeutics Market, by End-User |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US Lysosomal Storage Diseases Therapeutics Market, by Treatment Type |
|
8.2.10.1.2. US Lysosomal Storage Diseases Therapeutics Market, by Disease Type |
|
8.2.10.1.3. US Lysosomal Storage Diseases Therapeutics Market, by Route of Administration |
|
8.2.10.1.4. US Lysosomal Storage Diseases Therapeutics Market, by End-User |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Sanofi S.A. |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Takeda Pharmaceutical Company Limited |
|
10.3. BioMarin Pharmaceutical Inc. |
|
10.4. Pfizer Inc. |
|
10.5. Amicus Therapeutics, Inc. |
|
10.6. Alexion Pharmaceuticals, Inc. |
|
10.7. Ultragenyx Pharmaceutical Inc. |
|
10.8. Orchard Therapeutics plc |
|
10.9. Freeline Therapeutics |
|
10.10. Spark Therapeutics, Inc. |
|
10.11. JCR Pharmaceuticals Co., Ltd. |
|
10.12. Eli Lilly and Company |
|
10.13. Protalix BioTherapeutics, Inc. |
|
10.14. Horizon Therapeutics plc |
|
10.15. Sangamo Therapeutics, Inc. |
|
11. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Lysosomal Storage Diseases Therapeutics Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Lysosomal Storage Diseases Therapeutics Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Lysosomal Storage Diseases Therapeutics Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
