As per Intent Market Research, the Lyophilized Drugs Market was valued at USD 0.4 Billion in 2024-e and will surpass USD 0.6 Billion by 2030; growing at a CAGR of 8.1% during 2025-2030.
The global lyophilized drugs market is witnessing significant growth, driven by an increasing demand for biologics and the need for extended shelf life and improved stability of pharmaceutical products. Lyophilization, also known as freeze-drying, is a critical technology for preserving sensitive and temperature-sensitive drugs, especially biologics such as proteins, peptides, and monoclonal antibodies. The demand for lyophilized drugs is closely tied to the growing biologics sector, where formulations require enhanced stability, ease of transportation, and long shelf life. The market is poised for steady growth due to advancements in drug preservation techniques, the rising prevalence of chronic diseases, and the ever-growing biopharmaceutical sector.
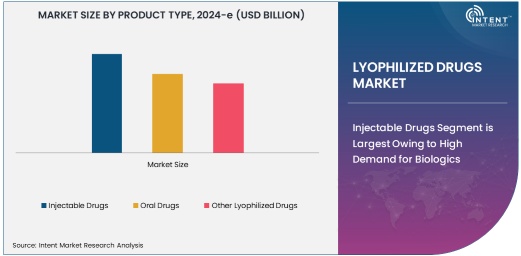
Injectable Drugs Segment is Largest Owing to High Demand for Biologics
The injectable drugs segment is the largest in the lyophilized drugs market, driven primarily by the growing demand for biologics in the pharmaceutical industry. Injectable lyophilized drugs are crucial in delivering high-precision treatments, particularly for chronic diseases and conditions that require long-term management, such as cancer and autoimmune disorders. The freeze-drying process allows injectable drugs to retain their potency, making them ideal for biologics that are sensitive to heat and moisture. With the increasing number of biologic drugs being approved, injectable drugs continue to dominate the market, offering pharmaceutical companies an effective and reliable means of delivering life-saving treatments.
The growing prevalence of chronic diseases, especially cancer, has further boosted the need for injectable biologics, which are often administered in hospital settings or specialty clinics. Additionally, the high cost and complexity of developing biologics increase the market's reliance on injectable forms, as they allow more efficient distribution and patient access. As more biologic therapies gain approval, injectable lyophilized drugs are expected to maintain their dominance, with further market expansion anticipated in the coming years.
Cancer Treatment Application is Fastest Growing Owing to Increased Incidence of Oncology Disorders
In the application segment, cancer treatment is the fastest-growing area for lyophilized drugs. The increasing global incidence of various types of cancer has spurred the demand for innovative therapies, particularly those involving biologics like monoclonal antibodies, which are often lyophilized to ensure stability and shelf life. Lyophilized cancer treatments, such as trastuzumab and rituximab, are gaining popularity due to their ease of storage and transportation, which is especially important in global distribution networks. The adoption of lyophilization in oncology treatments is not only increasing the shelf life but also enabling better outcomes by maintaining the potency of the drugs during storage and transportation.
As cancer care continues to evolve with targeted therapies and immunotherapies, the demand for stable, long-lasting lyophilized cancer treatments will only intensify. The ability to store and transport these treatments efficiently has become a crucial factor in ensuring that patients worldwide have access to cutting-edge oncology therapies. Given the global rise in cancer cases, this segment is expected to see continued growth, making it one of the most promising areas in the lyophilized drugs market.
Hospitals Segment is Largest End-User Owing to High Treatment Demands
Among the end-users, hospitals represent the largest segment in the lyophilized drugs market. Hospitals are the primary setting for the administration of injectable biologics, particularly for chronic and life-threatening conditions such as cancer, cardiovascular diseases, and autoimmune disorders. With the increasing number of patients requiring biologic therapies, hospitals are the primary consumers of lyophilized drugs. The ability to store lyophilized drugs for extended periods, without the need for refrigeration, makes them highly suited for hospital environments, where rapid deployment and efficient inventory management are essential.
Furthermore, hospitals are key players in the administration of advanced biologic treatments, especially in oncology and immunotherapy. With the continued advancement of healthcare infrastructures and the rise of specialized treatment centers, the hospital segment is expected to remain the largest end-user of lyophilized drugs. The robust healthcare system in developed regions, combined with the increasing number of cancer patients and chronic disease cases, is likely to drive this segment’s dominance in the market.
Vials Segment is Largest Packaging Type Owing to Versatility in Drug Distribution
In the packaging type segment, vials are the largest subsegment, owing to their versatility and widespread use in the pharmaceutical industry. Vials are particularly well-suited for the packaging of lyophilized drugs, as they can be sealed to prevent contamination and preserve the stability of the contents. The vials are typically used for injectable drugs and provide ease of use, ensuring that healthcare providers can easily administer the drug while maintaining its integrity. The global pharmaceutical industry relies heavily on vials for packaging lyophilized biologics, given their ability to withstand transportation and storage conditions.
The vials segment also benefits from advancements in packaging technologies, such as pre-filled vials that allow for more efficient and safer drug administration. With the increasing focus on biologics and injectable therapies, the demand for vials is expected to grow. Additionally, the shift towards self-administration and homecare settings further supports the continued dominance of vials in the lyophilized drugs market.
North America Region is Largest Owing to High Demand and Advanced Healthcare Infrastructure
North America is the largest region in the lyophilized drugs market, primarily driven by high demand for biologics, an advanced healthcare infrastructure, and a strong pharmaceutical industry presence. The United States, in particular, is a key market, home to some of the world’s leading pharmaceutical companies and research institutions. The region’s well-established healthcare system, coupled with a high prevalence of chronic diseases, ensures a steady demand for lyophilized drugs, especially in oncology and immunotherapy.
Moreover, the regulatory landscape in North America, which supports the development and approval of biologic drugs, further boosts the growth of the lyophilized drugs market. With increasing investments in healthcare and pharmaceuticals, North America is expected to maintain its position as the largest market for lyophilized drugs in the forecast period.
Competitive Landscape and Leading Companies
The lyophilized drugs market is highly competitive, with major pharmaceutical companies leading the charge in the development and distribution of freeze-dried drugs. Key players such as Pfizer Inc., Amgen Inc., and Roche Holding AG are heavily invested in the production and supply of lyophilized biologics. These companies have established a strong presence in the market by leveraging their research capabilities, extensive production networks, and regulatory expertise to bring lyophilized drugs to market.
In addition to these industry giants, contract manufacturers and specialized lyophilization service providers also play a significant role in the market. Companies such as Lonza Group, WuXi AppTec, and Patheon (Thermo Fisher Scientific) offer specialized lyophilization services, helping biopharmaceutical companies enhance the stability and shelf life of their drug products. As the demand for biologics continues to rise, the competitive landscape is expected to intensify, with companies focusing on strategic partnerships, technological advancements, and expanding production capacities to meet the growing global demand for lyophilized drugs.
Recent Developments:
- Pfizer announced the expansion of its lyophilization production capabilities to meet the rising demand for biologics and vaccines, particularly in emerging markets.
- Amgen received regulatory approval from the FDA for its new lyophilized version of a high-demand biologic drug, enhancing its distribution capabilities.
- Novartis has entered into a partnership with a leading lyophilization services provider to enhance its portfolio of vaccines and biologics.
- BMS announced a significant investment in lyophilization technology to support its expanding oncology drug portfolio.
- Roche launched a new lyophilized version of one of its oncology treatments, aimed at improving patient accessibility and reducing transportation and storage costs.
List of Leading Companies:
- Pfizer Inc.
- Bristol-Myers Squibb Company
- Amgen Inc.
- Eli Lilly and Company
- Roche Holding AG
- Novartis AG
- Merck & Co., Inc.
- Johnson & Johnson
- Sanofi S.A.
- AbbVie Inc.
- Bayer AG
- GlaxoSmithKline plc
- Teva Pharmaceutical Industries Ltd.
- Sandoz (Novartis)
- Mylan N.V.
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 0.4 Billion |
|
Forecasted Value (2030) |
USD 0.6 Billion |
|
CAGR (2025 – 2030) |
8.1% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Lyophilized Drugs Market By Product Type (Injectable Drugs, Oral Drugs, Lyophilized Drugs for Vaccines, Lyophilized Drugs for Proteins & Peptides, Lyophilized Drugs for Antibodies), By Application (Cancer Treatment, Cardiovascular Diseases, Infectious Diseases, Other Applications), By End-User (Hospitals, Clinics, Homecare Settings, Specialty Centers), By Packaging Type (Vials, Syringes, Other Packaging Types) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Pfizer Inc., Bristol-Myers Squibb Company, Amgen Inc., Eli Lilly and Company, Roche Holding AG, Novartis AG, Merck & Co., Inc., Johnson & Johnson, Sanofi S.A., AbbVie Inc., Bayer AG, GlaxoSmithKline plc, Teva Pharmaceutical Industries Ltd., Sandoz (Novartis), Mylan N.V. |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Lyophilized Drugs Market, by Product Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Injectable Drugs |
|
4.2. Oral Drugs |
|
4.3. Other Lyophilized Drugs |
|
5. Lyophilized Drugs Market, by Application (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Cancer Treatment |
|
5.2. Cardiovascular Diseases |
|
5.3. Infectious Diseases |
|
5.4. Other Applications |
|
6. Lyophilized Drugs Market, by End-User (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Hospitals |
|
6.2. Clinics |
|
6.3. Homecare Settings |
|
6.4. Specialty Centers |
|
6.5. Others |
|
7. Lyophilized Drugs Market, by Packaging Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Vials |
|
7.2. Syringes |
|
7.3. Other Packaging Types |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America Lyophilized Drugs Market, by Product Type |
|
8.2.7. North America Lyophilized Drugs Market, by Application |
|
8.2.8. North America Lyophilized Drugs Market, by End-User |
|
8.2.9. North America Lyophilized Drugs Market, by Packaging Type |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US Lyophilized Drugs Market, by Product Type |
|
8.2.10.1.2. US Lyophilized Drugs Market, by Application |
|
8.2.10.1.3. US Lyophilized Drugs Market, by End-User |
|
8.2.10.1.4. US Lyophilized Drugs Market, by Packaging Type |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Pfizer Inc. |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Bristol-Myers Squibb Company |
|
10.3. Amgen Inc. |
|
10.4. Eli Lilly and Company |
|
10.5. Roche Holding AG |
|
10.6. Novartis AG |
|
10.7. Merck & Co., Inc. |
|
10.8. Johnson & Johnson |
|
10.9. Sanofi S.A. |
|
10.10. AbbVie Inc. |
|
10.11. Bayer AG |
|
10.12. GlaxoSmithKline plc |
|
10.13. Teva Pharmaceutical Industries Ltd. |
|
10.14. Sandoz (Novartis) |
|
10.15. Mylan N.V. |
|
11. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Lyophilized Drugs Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Lyophilized Drugs Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Lyophilized Drugs Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
