As per Intent Market Research, the Intravenous Immunoglobulin Market was valued at USD 12.2 Billion in 2024-e and will surpass USD 20.1 Billion by 2030; growing at a CAGR of 8.6% during 2025 - 2030.
The intravenous immunoglobulin (IVIG) market is a crucial segment within the biopharmaceutical industry, driven by the increasing prevalence of immune-related disorders and the growing demand for effective treatments. IVIG is a blood product derived from human plasma, widely used in treating a variety of conditions, including primary immunodeficiencies, autoimmune diseases, and neurological disorders. The market’s growth is fueled by advancements in IVIG production technologies, expanding indications for its use, and the rising awareness of immune system disorders.
As healthcare systems around the world continue to prioritize advanced treatments, IVIG products are becoming indispensable in managing conditions that affect the immune system. The global demand for IVIG is expected to increase, particularly in regions with aging populations and high incidences of autoimmune diseases. This market is characterized by a steady shift towards targeted therapies, with significant investments in improving the efficacy and safety profiles of IVIG products.
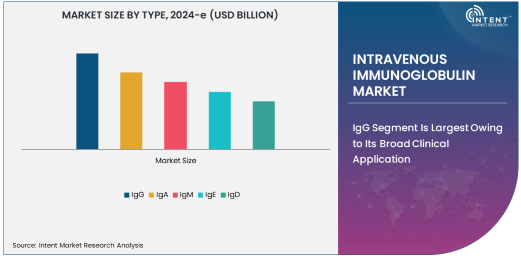
IgG Segment Is Largest Owing to Its Broad Clinical Application
The IgG (Immunoglobulin G) segment is the largest in the intravenous immunoglobulin market, owing to its widespread clinical application and effectiveness in treating a range of immune-related diseases. IgG is the most abundant antibody in human blood and is crucial in providing passive immunity. IVIG prepared from IgG is commonly used to treat primary immunodeficiencies, autoimmune diseases, and neurological disorders such as Chronic Inflammatory Demyelinating Polyneuropathy (CIDP). The versatility of IgG-based IVIG products, combined with their established track record in medical treatments, positions them as the dominant segment in the market.
The large-scale use of IgG IVIG is also attributed to its ability to modulate immune system activity, making it effective for patients with conditions that involve immune dysfunction. Furthermore, with the increasing diagnosis of primary immunodeficiency and autoimmune diseases globally, the demand for IgG IVIG continues to rise, solidifying its position as the largest subsegment within the IVIG market. As the global healthcare infrastructure expands, especially in emerging markets, IgG-based therapies are expected to maintain their dominant role in treating complex immune disorders.
Primary Immunodeficiency Segment Is Fastest Growing Owing to Increased Diagnosis
The primary immunodeficiency (PID) segment is the fastest growing in the intravenous immunoglobulin market, driven by the rising global diagnosis rates and advancements in genetic testing. Primary immunodeficiencies are a group of disorders where the immune system's ability to fight infections is impaired. IVIG therapy plays a central role in the management of PID, particularly in individuals with severe combined immunodeficiency (SCID) or common variable immunodeficiency (CVID), by providing essential antibodies to boost the patient’s immune response. The increasing recognition of PID as a treatable condition is spurring growth in this segment.
As healthcare providers become more aware of the clinical signs of PID, and as diagnostic tools become more accessible, more patients are being identified and treated with IVIG. This shift towards earlier diagnosis and proactive treatment is contributing to the rapid expansion of the primary immunodeficiency segment. With greater investments in research and a growing understanding of the genetic factors behind PID, this subsegment is poised for continued growth, especially in developed markets with advanced healthcare systems.
Hospitals Segment Is Largest Owing to High Demand for Critical Care
The hospitals segment is the largest end-use industry in the intravenous immunoglobulin market, driven by the high demand for IVIG therapies in acute care settings. Hospitals are the primary healthcare facilities where patients with severe immune disorders, neurological diseases, and autoimmune conditions are treated. The critical nature of the conditions managed with IVIG, such as CIDP, Myasthenia Gravis, and primary immunodeficiencies, leads to a significant demand for these therapies in hospital settings. Additionally, hospitals are better equipped to handle the complex administration of IVIG, which often requires specialized staff and equipment for intravenous infusion.
The strong presence of hospitals as the preferred setting for administering IVIG therapies is bolstered by the need for immediate, intensive care for patients with compromised immune systems. Hospitals also benefit from access to comprehensive diagnostic and treatment services, which enhance patient outcomes. With the rise in the incidence of diseases requiring immunoglobulin therapy, hospitals remain the largest consumer of IVIG products, accounting for a significant share of the market.
North America Region Is Largest Owing to High Healthcare Expenditure
North America is the largest region in the intravenous immunoglobulin market, owing to high healthcare expenditures, advanced medical infrastructure, and a growing prevalence of autoimmune diseases and immune-related disorders. The region, particularly the United States, has a well-established healthcare system that provides broad access to IVIG therapies for treating various conditions, including primary immunodeficiencies and autoimmune disorders. The high adoption rate of IVIG treatments in both hospitals and specialized clinics, coupled with extensive insurance coverage for these therapies, contributes to North America’s dominance in the global IVIG market.
Furthermore, the United States is home to several key players in the pharmaceutical and biotechnology industries, which facilitates the continuous development of advanced IVIG formulations and production techniques. The region’s strong healthcare infrastructure, combined with the increasing awareness and diagnosis of conditions requiring IVIG, ensures that North America will continue to lead the market in terms of both demand and innovation.

Competitive Landscape and Key Players
The competitive landscape in the intravenous immunoglobulin market is marked by the presence of a few global leaders who dominate the production and distribution of IVIG products. Key players such as Grifols, CSL Behring, Shire (now part of Takeda), and Octapharma are at the forefront of the market, offering a range of IVIG therapies for various indications. These companies have a strong market presence due to their extensive product portfolios, robust distribution networks, and significant investment in research and development.
The market is highly competitive, with companies focusing on enhancing the purity, safety, and efficacy of their IVIG products to meet the growing demand for personalized treatments. Companies are also exploring innovative delivery methods, such as subcutaneous administration, to improve patient convenience and compliance. As the market continues to expand, mergers, acquisitions, and strategic partnerships will likely intensify as companies seek to strengthen their market position, expand their product offerings, and enter emerging markets. The ongoing innovation in IVIG formulations and therapies will continue to shape the competitive dynamics of the market.
Recent Developments:
- CSL Behring expanded its portfolio by launching a new IVIG product targeting CIDP patients in Europe.
- Grifols, S.A. announced the acquisition of a plasma fractionation facility to boost its IVIG production capacity.
- Takeda Pharmaceutical unveiled data on the efficacy of its IVIG product in treating rare autoimmune disorders.
- Octapharma AG introduced a novel IVIG formulation with improved infusion tolerability.
- Kedrion Biopharma entered a strategic partnership with a biotechnology company to enhance plasma-derived therapies.
List of Leading Companies:
- CSL Behring
- Grifols, S.A.
- Takeda Pharmaceutical Company Limited
- Kedrion Biopharma
- Octapharma AG
- Bio Products Laboratory (BPL)
- LFB Group
- Shanghai RAAS Blood Products Co., Ltd.
- Baxalta (A Takeda Company)
- China Biologic Products Holdings, Inc.
- Sanquin Blood Supply Foundation
- Emergent BioSolutions Inc.
- Biotest AG
- Hualan Biological Engineering Inc.
- ADMA Biologics, Inc.
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 12.2 Billion |
|
Forecasted Value (2030) |
USD 20.1 Billion |
|
CAGR (2025 – 2030) |
8.6% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Intravenous Immunoglobulin Market by Type (IgG, IgA, IgM, IgE, IgD), by Application (Primary Immunodeficiency, Chronic Inflammatory Demyelinating Polyneuropathy, Myasthenia Gravis), by End-Use Industry (Hospitals, Clinics), and by Region |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
CSL Behring, Grifols, S.A., Takeda Pharmaceutical Company Limited, Kedrion Biopharma, Octapharma AG, Bio Products Laboratory (BPL), Shanghai RAAS Blood Products Co., Ltd., Baxalta (A Takeda Company), China Biologic Products Holdings, Inc., Sanquin Blood Supply Foundation, Emergent BioSolutions Inc., Biotest AG, ADMA Biologics, Inc. |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Intravenous Immunoglobulin Market, by Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. IgG |
|
4.2. IgA |
|
4.3. IgM |
|
4.4. IgE |
|
4.5. IgD |
|
5. Intravenous Immunoglobulin Market, by Application (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Primary Immunodeficiency |
|
5.2. Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) |
|
5.3. Myasthenia Gravis |
|
5.4. Others |
|
6. Intravenous Immunoglobulin Market, by End-Use Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Hospitals |
|
6.2. Clinics |
|
6.3. Others |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Intravenous Immunoglobulin Market, by Type |
|
7.2.7. North America Intravenous Immunoglobulin Market, by Application |
|
7.2.8. North America Intravenous Immunoglobulin Market, by End-Use Industry |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Intravenous Immunoglobulin Market, by Type |
|
7.2.9.1.2. US Intravenous Immunoglobulin Market, by Application |
|
7.2.9.1.3. US Intravenous Immunoglobulin Market, by End-Use Industry |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. CSL Behring |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. Grifols, S.A. |
|
9.3. Takeda Pharmaceutical Company Limited |
|
9.4. Kedrion Biopharma |
|
9.5. Octapharma AG |
|
9.6. Bio Products Laboratory (BPL) |
|
9.7. LFB Group |
|
9.8. Shanghai RAAS Blood Products Co., Ltd. |
|
9.9. Baxalta (A Takeda Company) |
|
9.10. China Biologic Products Holdings, Inc. |
|
9.11. Sanquin Blood Supply Foundation |
|
9.12. Emergent BioSolutions Inc. |
|
9.13. Biotest AG |
|
9.14. Hualan Biological Engineering Inc. |
|
9.15. ADMA Biologics, Inc. |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Intravenous Immunoglobulin Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Intravenous Immunoglobulin Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Intravenous Immunoglobulin Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
