As per Intent Market Research, the Interventional Neurology Devices Market was valued at USD 2.6 Billion in 2024-e and will surpass USD 4.3 Billion by 2030; growing at a CAGR of 8.6% during 2025 - 2030.
The Interventional Neurology Devices Market is witnessing significant growth due to the rising incidence of neurological disorders such as strokes, aneurysms, and cerebral artery diseases. These devices play a crucial role in minimally invasive procedures aimed at treating a variety of neurological conditions, offering reduced recovery times and lower risks compared to traditional surgical methods. As advancements in medical technology continue to enhance the precision and effectiveness of these devices, the demand for interventional neurology solutions is expected to grow steadily.
Key drivers of this market include an aging global population, increased prevalence of cerebrovascular diseases, and the growing adoption of minimally invasive surgical techniques. Moreover, innovation in neurovascular technologies, including the development of flow diverters and thrombectomy devices, continues to create new opportunities for market growth.
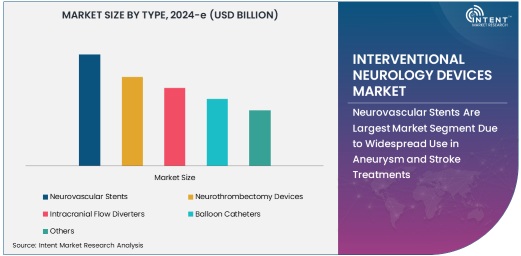
Neurovascular Stents Are Largest Market Segment Due to Widespread Use in Aneurysm and Stroke Treatments
Neurovascular stents represent the largest segment in the interventional neurology devices market, owing to their extensive use in treating cerebrovascular diseases, including aneurysms and ischemic strokes. These stents are critical for maintaining vascular patency and improving blood flow in the brain during interventions such as stent-assisted coil embolization. The ability of neurovascular stents to prevent vessel collapse and improve outcomes in high-risk patients has made them an essential component of interventional neurology.
As the incidence of aneurysms and ischemic strokes continues to rise, the demand for neurovascular stents is projected to grow. Moreover, the development of self-expanding and bioresorbable stents is driving innovation in this market, offering better patient outcomes and fewer complications. The increasing adoption of these devices in emergency settings, particularly in stroke care, is also contributing to the segment's leadership.
Neurothrombectomy Devices Are Fastest-Growing Segment Due to Advancements in Stroke Treatment
Neurothrombectomy devices are the fastest-growing segment in the interventional neurology devices market, primarily driven by the increasing prevalence of ischemic strokes and advancements in stroke treatment technologies. These devices are essential for the mechanical removal of blood clots from cerebral arteries, a procedure that has been shown to significantly improve outcomes in patients with acute ischemic stroke.
Technological improvements, such as the development of advanced thrombectomy devices with better clot capture and removal capabilities, have made these devices increasingly effective. The growth of neurothrombectomy devices is further fueled by clinical evidence supporting their use in acute stroke interventions, as well as their expanded application in hospital settings worldwide.
Aneurysm Treatment Leads the Application Segment Due to High Incidence of Cerebral Aneurysms
Aneurysm treatment is the largest application segment within the interventional neurology devices market, largely due to the high prevalence of cerebral aneurysms. These conditions require urgent medical intervention to prevent rupture, which can lead to life-threatening complications such as strokes and brain hemorrhages. Devices such as neurovascular stents and coils are commonly used to treat aneurysms through embolization techniques, making this a key focus area for interventional neurology.
The increasing adoption of minimally invasive procedures for aneurysm treatment, driven by their ability to reduce recovery times and improve patient outcomes, has further boosted the demand for related devices. As awareness of early detection and treatment of cerebral aneurysms rises, the need for advanced interventional neurology solutions in this area continues to expand.
Hospitals Dominate the End-Use Industry Due to High Procedure Volumes and Advanced Technology Availability
Hospitals represent the largest end-use industry segment for interventional neurology devices, driven by their capacity to perform complex neurological procedures using the most advanced technologies. Hospitals are the primary settings for the treatment of cerebrovascular diseases such as ischemic strokes, aneurysms, and cerebral artery diseases, all of which require specialized equipment and skilled medical personnel.
Furthermore, hospitals are increasingly investing in state-of-the-art neurovascular imaging systems and interventional devices, enabling them to provide high-quality care to patients. The rising number of hospitals equipped with dedicated neurointerventional units and the growing focus on improving neurological care standards globally further support the segment's dominance.
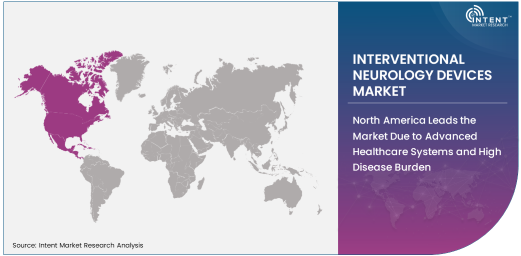
North America Leads the Market Due to Advanced Healthcare Systems and High Disease Burden
North America is the largest regional market for interventional neurology devices, primarily driven by the high incidence of neurological disorders and the presence of advanced healthcare infrastructure. The United States, in particular, leads the region due to its strong healthcare system, high adoption rates of advanced medical technologies, and well-established neurointerventional care programs.
Moreover, the significant investments in research and development of new interventional devices, coupled with government initiatives to improve stroke care and the treatment of neurological disorders, further bolster the growth of this market. The rising awareness of stroke prevention and the increasing availability of neurointerventional procedures also contribute to North America’s leading position.
Competitive Landscape and Leading Companies
The Interventional Neurology Devices Market is highly competitive, with several global players dominating the space. Major companies in this market include Medtronic, Stryker Corporation, Boston Scientific Corporation, Terumo Corporation, and Johnson & Johnson. These companies are focusing on developing next-generation devices, incorporating advanced materials and technologies to enhance device performance and patient outcomes.
The market is also witnessing increased collaboration between device manufacturers, hospitals, and research institutions to develop and refine interventional neurology devices. With the growing demand for minimally invasive treatments, companies are investing heavily in innovation, particularly in the areas of neurovascular stents, thrombectomy devices, and cerebral flow diverters. As the incidence of stroke and aneurysms continues to rise, the competitive landscape is expected to remain dynamic, with companies aiming to expand their market share through strategic acquisitions, product innovations, and regional expansion.
Recent Developments:
- Medtronic launched a new neurovascular stent designed for complex aneurysm cases, improving treatment precision.
- Stryker Corporation received FDA approval for their latest thrombectomy device to treat ischemic strokes.
- Abbott Laboratories expanded its interventional neurology portfolio with a next-generation balloon catheter for carotid artery treatment.
- Boston Scientific introduced a new intracranial flow diverter aimed at treating complex brain aneurysms.
- Penumbra, Inc. announced the release of a new embolic coil device for treating large vessel occlusion strokes.
List of Leading Companies:
- Medtronic PLC
- Stryker Corporation
- Johnson & Johnson (Cerenovus)
- Terumo Corporation
- Abbott Laboratories
- Boston Scientific Corporation
- Penumbra, Inc.
- MicroPort Scientific Corporation
- Aneurysm Coils
- Neurovasc Technologies
- Nico Corporation
- InspireMD, Inc.
- Asahi Intecc Co., Ltd.
- Merit Medical Systems
- St. Jude Medical (now part of Abbott)
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 2.6 Billion |
|
Forecasted Value (2030) |
USD 4.3 Billion |
|
CAGR (2025 – 2030) |
8.6% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Global Interventional Neurology Devices Market by Type (Neurovascular Stents, Neurothrombectomy Devices, Intracranial Flow Diverters, Balloon Catheters), by Application (Aneurysm Treatment, Ischemic Stroke Treatment, Cerebral Artery Disease Treatment), by End-Use Industry (Hospitals, Ambulatory Surgery Centers, Specialty Clinics), and by Region |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Medtronic PLC, Stryker Corporation, Johnson & Johnson (Cerenovus), Terumo Corporation, Abbott Laboratories, Boston Scientific Corporation, MicroPort Scientific Corporation, Aneurysm Coils, Neurovasc Technologies, Nico Corporation, InspireMD, Inc., Asahi Intecc Co., Ltd., St. Jude Medical (now part of Abbott) |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Interventional Neurology Devices Market, by Type (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Neurovascular Stents |
|
4.2. Neurothrombectomy Devices |
|
4.3. Intracranial Flow Diverters |
|
4.4. Balloon Catheters |
|
4.5. Others |
|
5. Interventional Neurology Devices Market, by Application (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. Aneurysm Treatment |
|
5.2. Ischemic Stroke Treatment |
|
5.3. Cerebral Artery Disease Treatment |
|
5.4. Others |
|
6. Interventional Neurology Devices Market, by End-Use Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Hospitals |
|
6.2. Ambulatory Surgery Centers |
|
6.3. Specialty Clinics |
|
7. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Regional Overview |
|
7.2. North America |
|
7.2.1. Regional Trends & Growth Drivers |
|
7.2.2. Barriers & Challenges |
|
7.2.3. Opportunities |
|
7.2.4. Factor Impact Analysis |
|
7.2.5. Technology Trends |
|
7.2.6. North America Interventional Neurology Devices Market, by Type |
|
7.2.7. North America Interventional Neurology Devices Market, by Application |
|
7.2.8. North America Interventional Neurology Devices Market, by End-Use Industry |
|
7.2.9. By Country |
|
7.2.9.1. US |
|
7.2.9.1.1. US Interventional Neurology Devices Market, by Type |
|
7.2.9.1.2. US Interventional Neurology Devices Market, by Application |
|
7.2.9.1.3. US Interventional Neurology Devices Market, by End-Use Industry |
|
7.2.9.2. Canada |
|
7.2.9.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
7.3. Europe |
|
7.4. Asia-Pacific |
|
7.5. Latin America |
|
7.6. Middle East & Africa |
|
8. Competitive Landscape |
|
8.1. Overview of the Key Players |
|
8.2. Competitive Ecosystem |
|
8.2.1. Level of Fragmentation |
|
8.2.2. Market Consolidation |
|
8.2.3. Product Innovation |
|
8.3. Company Share Analysis |
|
8.4. Company Benchmarking Matrix |
|
8.4.1. Strategic Overview |
|
8.4.2. Product Innovations |
|
8.5. Start-up Ecosystem |
|
8.6. Strategic Competitive Insights/ Customer Imperatives |
|
8.7. ESG Matrix/ Sustainability Matrix |
|
8.8. Manufacturing Network |
|
8.8.1. Locations |
|
8.8.2. Supply Chain and Logistics |
|
8.8.3. Product Flexibility/Customization |
|
8.8.4. Digital Transformation and Connectivity |
|
8.8.5. Environmental and Regulatory Compliance |
|
8.9. Technology Readiness Level Matrix |
|
8.10. Technology Maturity Curve |
|
8.11. Buying Criteria |
|
9. Company Profiles |
|
9.1. Medtronic PLC |
|
9.1.1. Company Overview |
|
9.1.2. Company Financials |
|
9.1.3. Product/Service Portfolio |
|
9.1.4. Recent Developments |
|
9.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
9.2. Stryker Corporation |
|
9.3. Johnson & Johnson (Cerenovus) |
|
9.4. Terumo Corporation |
|
9.5. Abbott Laboratories |
|
9.6. Boston Scientific Corporation |
|
9.7. Penumbra, Inc. |
|
9.8. MicroPort Scientific Corporation |
|
9.9. Aneurysm Coils |
|
9.10. Neurovasc Technologies |
|
9.11. Nico Corporation |
|
9.12. InspireMD, Inc. |
|
9.13. Asahi Intecc Co., Ltd. |
|
9.14. Merit Medical Systems |
|
9.15. St. Jude Medical (now part of Abbott) |
|
10. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Interventional Neurology Devices Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Interventional Neurology Devices Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Interventional Neurology Devices Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
