As per Intent Market Research, the Infectious Disease Diagnostics Market was valued at USD 2.3 Billion in 2024-e and will surpass USD 6.0 Billion by 2030; growing at a CAGR of 17.3% during 2025 - 2030.
The Infectious Disease Diagnostics market has become a cornerstone in global healthcare, particularly as the world continues to combat an array of infectious diseases. The growing prevalence of infectious diseases, along with the rise of new pathogens and antibiotic resistance, is significantly driving the demand for advanced diagnostic solutions. With an increasing emphasis on early diagnosis, precise detection, and rapid response, diagnostic tools have evolved to ensure timely treatment and control over the spread of infections. Immunoassays, molecular diagnostics, and point-of-care (POC) testing are some of the primary products used for diagnosing a range of infectious diseases.
The market is expected to grow rapidly as healthcare providers, hospitals, and diagnostic laboratories integrate new technologies for infection detection. Additionally, the increasing adoption of personalized medicine and a rising focus on preventive healthcare further push the need for accurate diagnostics. Moreover, advancements in diagnostic technologies, such as PCR-based diagnostics and enzyme-linked immunosorbent assays (ELISA), are continuously improving the precision and speed of infection detection, thereby facilitating quicker decision-making in patient care.
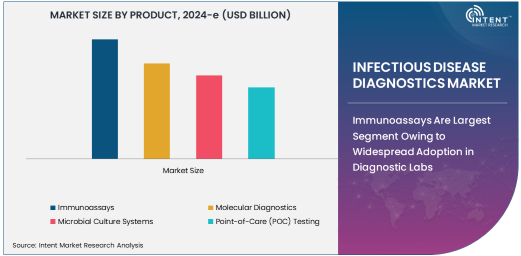
Immunoassays Are Largest Segment Owing to Widespread Adoption in Diagnostic Labs
Immunoassays are the largest segment in the infectious disease diagnostics market, due to their extensive use in diagnostic laboratories and healthcare settings. Immunoassays, which include methods like enzyme-linked immunosorbent assay (ELISA), are widely used for detecting and quantifying specific infectious disease markers, such as antibodies or antigens, in blood or other bodily fluids. These diagnostic tests are particularly beneficial due to their high sensitivity, ease of use, and cost-effectiveness, making them suitable for a variety of infectious diseases, including viral, bacterial, and parasitic infections.
The popularity of immunoassays can be attributed to their broad range of applications across infectious diseases like HIV, Hepatitis, and influenza. Their ability to provide fast results and be deployed in high-throughput laboratory environments has cemented their position as a key tool in disease surveillance, diagnosis, and epidemiological studies. As the demand for rapid and accurate diagnostic solutions continues to rise, immunoassays will remain the dominant diagnostic technology in the infectious disease diagnostics market.
Molecular Diagnostics Is Fastest Growing Segment Owing to Advances in Technology
Molecular diagnostics represent the fastest growing segment in the infectious disease diagnostics market, primarily driven by advances in technology that enable highly sensitive and specific tests. This category includes methods such as PCR-based diagnostics and next-generation sequencing (NGS), which allow for the detection of genetic material from pathogens, making it possible to identify infections at a molecular level. The precision of molecular diagnostics is particularly beneficial for detecting infections caused by pathogens that are difficult to identify using traditional culture methods.
The growth of molecular diagnostics is fueled by their ability to offer rapid, highly accurate results, even for hard-to-culture organisms, enabling faster diagnosis and timely intervention. The increasing prevalence of infectious diseases, the rise in demand for precision medicine, and the ongoing advancements in diagnostic technologies are expected to further accelerate the adoption of molecular diagnostics in clinical settings. As these technologies become more accessible and affordable, their use in both hospitals and point-of-care settings is expected to surge, making molecular diagnostics one of the key growth drivers in the infectious disease diagnostics market.
Hospitals and Diagnostic Laboratories Are Largest End-Use Industry Due to High Testing Volume
Hospitals and diagnostic laboratories are the largest end-use industry for infectious disease diagnostics due to their central role in disease detection and management. Hospitals are critical in diagnosing, treating, and managing infectious diseases, and diagnostic laboratories play a crucial part in providing accurate and timely test results. The large volume of diagnostic tests conducted in hospitals, especially for respiratory infections, gastrointestinal infections, and bloodstream infections, makes them the primary end-users of infectious disease diagnostics products.
Moreover, with the rise of multi-drug-resistant infections and the growing emphasis on patient safety, hospitals and diagnostic laboratories are increasingly adopting advanced diagnostic technologies to ensure the accurate and rapid detection of infections. The ability to swiftly identify pathogens enables healthcare professionals to prescribe the most appropriate treatments, reducing the risk of complications and improving patient outcomes. As a result, hospitals and diagnostic laboratories continue to drive the demand for a broad range of diagnostic tools, making this segment the largest in the market.
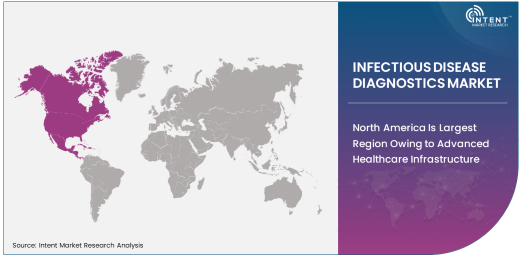
North America Is Largest Region Owing to Advanced Healthcare Infrastructure
North America is the largest region in the infectious disease diagnostics market, primarily due to its advanced healthcare infrastructure, high adoption of diagnostic technologies, and robust healthcare spending. The United States, in particular, leads the market, with a well-established healthcare system that supports a wide range of diagnostic procedures. The prevalence of infectious diseases, along with the high volume of diagnostic testing required for disease management, makes North America a major market for infectious disease diagnostics.
Additionally, the region is home to numerous leading healthcare companies and research institutions that drive innovations in diagnostic technologies, including PCR-based diagnostics and immunoassays. The regulatory environment in North America also supports the adoption of new and advanced diagnostic solutions, encouraging rapid market growth. With the increasing focus on healthcare quality, early detection of infectious diseases, and efficient disease management, North America is expected to maintain its dominant position in the infectious disease diagnostics market.
Leading Companies and Competitive Landscape
The infectious disease diagnostics market is highly competitive, with numerous companies providing a wide range of diagnostic solutions. Key players in the market include Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, Siemens Healthineers, BD (Becton, Dickinson and Company), and Cepheid. These companies are at the forefront of innovation, offering advanced products like immunoassays, molecular diagnostics, and point-of-care testing solutions.
The competitive landscape is characterized by ongoing research and development, strategic partnerships, and the continuous launch of new products. Companies are focusing on expanding their product portfolios to include more rapid, accurate, and cost-effective diagnostic tools. Furthermore, partnerships with healthcare providers, academic institutions, and government health agencies are key to enhancing the reach and effectiveness of these solutions. As the market for infectious disease diagnostics continues to grow, these leading companies are expected to maintain their competitive advantage by investing in next-generation diagnostic technologies and improving testing efficiencies.
Recent Developments:
- Thermo Fisher Scientific launched a new molecular diagnostic platform for faster and more accurate detection of respiratory infections, expanding its portfolio of infectious disease diagnostics.
- Roche Diagnostics received FDA approval for a new PCR-based diagnostic test that helps detect multiple pathogens in a single test for gastrointestinal infections.
- Danaher Corporation acquired a major player in the rapid testing space to enhance its diagnostic offerings for infectious diseases.
- Becton, Dickinson and Company introduced an upgraded version of its blood culture system, designed for faster identification of bloodstream infections.
- BioMérieux SA announced a strategic partnership with a global healthcare provider to improve infectious disease testing capabilities in underserved regions.
List of Leading Companies:
- Abbott Laboratories
- Roche Diagnostics
- Thermo Fisher Scientific
- Siemens Healthineers
- Becton, Dickinson and Company
- Hologic, Inc.
- Danaher Corporation
- BioMérieux SA
- Cepheid
- QIAGEN N.V.
- Grifols, S.A.
- Illumina, Inc.
- F. Hoffmann-La Roche Ltd.
- Alere, Inc. (acquired by Abbott)
- PerkinElmer, Inc.
Report Scope:
|
Report Features |
Description |
|
Market Size (2024-e) |
USD 2.3 Billion |
|
Forecasted Value (2030) |
USD 6.0 Billion |
|
CAGR (2025 – 2030) |
17.3% |
|
Base Year for Estimation |
2024-e |
|
Historic Year |
2023 |
|
Forecast Period |
2025 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Infectious Disease Diagnostics Market by Product (Immunoassays, Molecular Diagnostics, Microbial Culture Systems, Point-of-Care (POC) Testing); Technology (PCR-Based Diagnostics, Immunohistochemistry, Enzyme-Linked Immunosorbent Assay (ELISA)); Application (Respiratory Infections, Bloodstream Infections, Gastrointestinal Infections); End-Use Industry (Hospitals and Diagnostic Laboratories, Homecare and Point-of-Care Testing) and by Region |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, Siemens Healthineers, Becton, Dickinson and Company, Hologic, Inc., BioMérieux SA, Cepheid, QIAGEN N.V., Grifols, S.A., Illumina, Inc., F. Hoffmann-La Roche Ltd., PerkinElmer, Inc.
|
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Infectious Disease Diagnostics Market, by Product (Market Size & Forecast: USD Million, 2023 – 2030) |
|
4.1. Immunoassays |
|
4.2. Molecular Diagnostics |
|
4.3. Microbial Culture Systems |
|
4.4. Point-of-Care (POC) Testing |
|
5. Infectious Disease Diagnostics Market, by Technology (Market Size & Forecast: USD Million, 2023 – 2030) |
|
5.1. PCR-Based Diagnostics |
|
5.2. Immunohistochemistry |
|
5.3. Enzyme-Linked Immunosorbent Assay (ELISA) |
|
6. Infectious Disease Diagnostics Market, by Application (Market Size & Forecast: USD Million, 2023 – 2030) |
|
6.1. Respiratory Infections |
|
6.2. Bloodstream Infections |
|
6.3. Gastrointestinal Infections |
|
6.4. Others |
|
7. Infectious Disease Diagnostics Market, by End-Use Industry (Market Size & Forecast: USD Million, 2023 – 2030) |
|
7.1. Hospitals and Diagnostic Laboratories |
|
7.2. Homecare and Point-of-Care Testing |
|
7.3. Others |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2023 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America Infectious Disease Diagnostics Market, by Product |
|
8.2.7. North America Infectious Disease Diagnostics Market, by Technology |
|
8.2.8. North America Infectious Disease Diagnostics Market, by Application |
|
8.2.9. North America Infectious Disease Diagnostics Market, by End-Use Industry |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US Infectious Disease Diagnostics Market, by Product |
|
8.2.10.1.2. US Infectious Disease Diagnostics Market, by Technology |
|
8.2.10.1.3. US Infectious Disease Diagnostics Market, by Application |
|
8.2.10.1.4. US Infectious Disease Diagnostics Market, by End-Use Industry |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Abbott Laboratories |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Roche Diagnostics |
|
10.3. Thermo Fisher Scientific |
|
10.4. Siemens Healthineers |
|
10.5. Becton, Dickinson and Company |
|
10.6. Hologic, Inc. |
|
10.7. Danaher Corporation |
|
10.8. BioMérieux SA |
|
10.9. Cepheid |
|
10.10. QIAGEN N.V. |
|
10.11. Grifols, S.A. |
|
10.12. Illumina, Inc. |
|
10.13. F. Hoffmann-La Roche Ltd. |
|
10.14. Alere, Inc. (acquired by Abbott) |
|
10.15. PerkinElmer, Inc. |
|
11. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Infectious Disease Diagnostics Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Infectious Disease Diagnostics Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Infectious Disease Diagnostics Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
