sales@intentmarketresearch.com
+1 463-583-2713
eClinical Solutions Market By Solution (Clinical Trial Management Systems, Electronic Data Capture, Randomization and Trial Supply Management, Clinical Analytics Solutions, Regulatory Compliance Solutions, Patient Engagement Solutions), By Deployment Model (Cloud-Based Model, On-Premise Model), By Clinical Trial Phase (Phase I, Phase II, Phase III, Phase IV), and By End User (Pharmaceutical & Biopharmaceutical Companies, Contract Research Organizations, Hospitals & Healthcare Providers) and By Region; Global Insights & Forecast (2024 – 2030)
As per Intent Market Research, the eClinical Solutions Market was valued at USD 11.4 billion in 2023 and will surpass USD 23.3 billion by 2030; growing at a CAGR of 10.8% during 2024 - 2030.
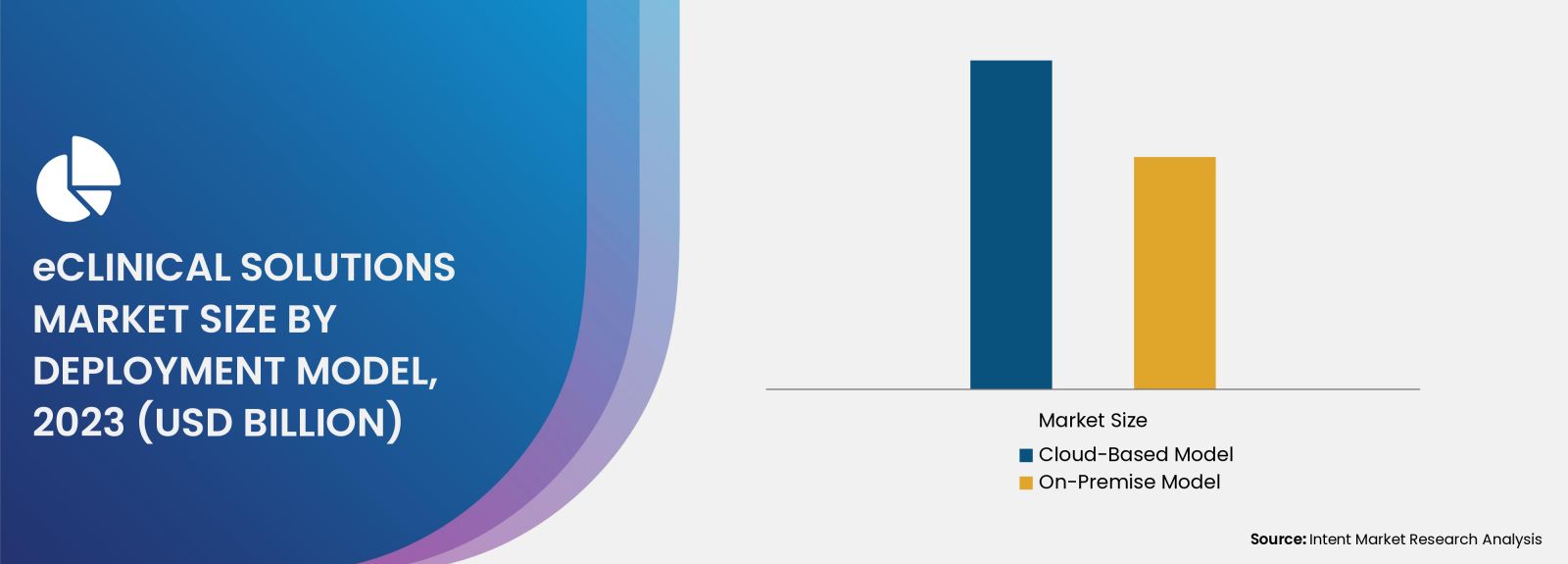
The Cloud-Based Model to be the Largest Segment in the eClinical Solutions Market
The dominance of cloud-based model can be attributed to several factors, including seamless integration with existing infrastructure without substantial capital investment, as well as enhanced flexibility and accessibility that enable researchers, clinicians, and other stakeholders to access data and applications from any location, thereby fostering greater collaboration and efficiency. Furthermore, cloud-based systems incorporate advanced security features and automatic updates, ensuring compliance with the latest regulations and protection against data breaches. These attributes contribute to their increased share in the eClinical solutions market.
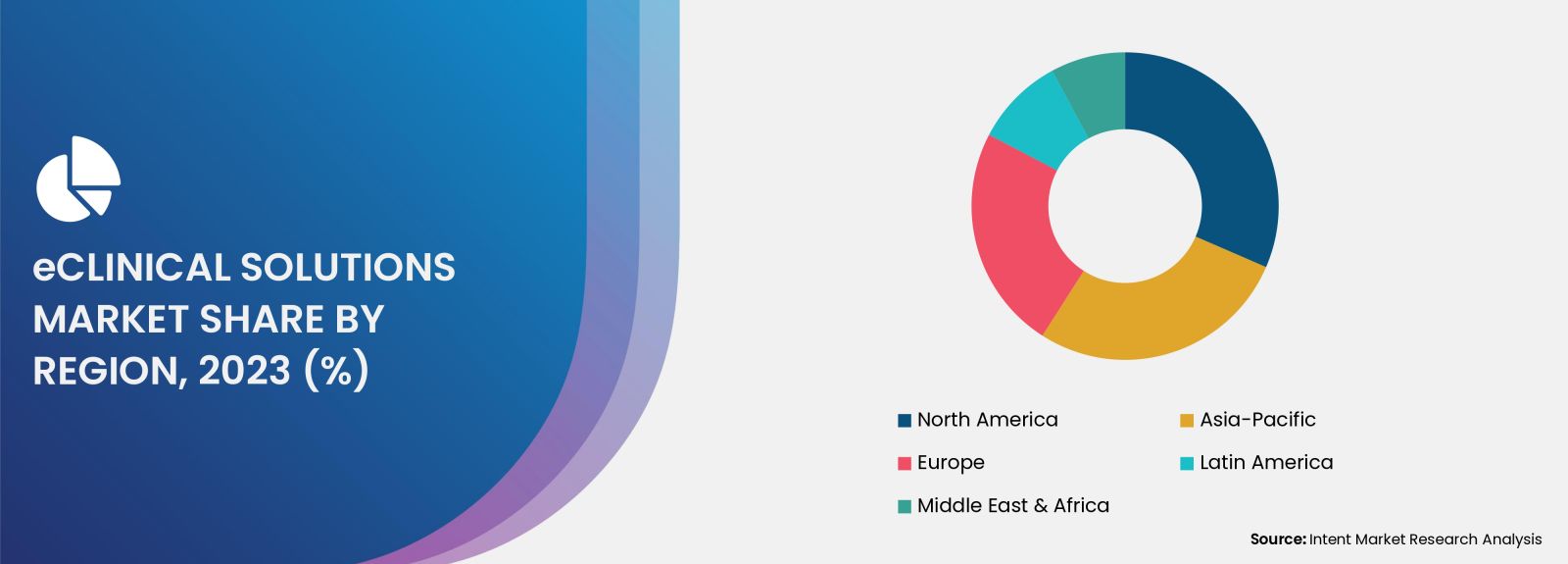
North America Accounted for the Largest Share of the eClinical Solutions Market
The prominence of North America can be attributed to the region's high levels of digital literacy and its commitment to technological innovation, as evidenced by the advancements made by leading companies such as Medidata Solutions. Additionally, the presence of robust regulatory frameworks, including the FDA's 21 CFR Part 11, enhances compliance measures, thereby fostering a greater demand for advanced data management and clinical trial solutions.
This regulatory support not only facilitates the implementation of eClinical solutions but also instills confidence among stakeholders in the integrity and security of clinical data management processes. As a result, North America continues to lead the way in the adoption and utilization of eClinical solutions, setting a precedent for other regions to follow.
The report focuses on estimating the current market potential in terms of the total addressable market for all the segments, sub-segments, and regions. In the process, all the high-growth and upcoming technologies were identified and analyzed to measure their impact on the current and future market. The report also identifies the key stakeholders, their business gaps, and their purchasing behavior. This information is essential for developing effective marketing strategies and creating products or services that meet the needs of the target market. The report also covers a detailed analysis of the competitive landscape which includes major players, their recent developments, growth strategies, product benchmarking, and manufacturing operations among others. Also, brief insights on start-up ecosystem and emerging companies is also included as part of this report.
Report Objectives:
The report will help you answer some of the most critical questions in the eClinical Solutions Market. A few of them are as follows:
- What are the key drivers, restraints, opportunities, and challenges influencing the market growth?
- What are the prevailing technology trends in the eClinical Solutions Market?
- What is the size of the eClinical Solutions Market based on segments, sub-segments, and regions?
- What is the size of different market segments across key regions: North America, Europe, Asia-Pacific, Latin America, Middle East & Africa?
- What are the market opportunities for stakeholders after analyzing key market trends?
- Who are the leading market players and what are their market share and core competencies?
- What is the degree of competition in the market and what are the key growth strategies adopted by leading players?
- What is the competitive landscape of the market, including market share analysis, revenue analysis, and a ranking of key players?
Report Scope:
|
Report Features |
Description |
|
Market Size (2023) |
USD 11.4 billion |
|
Forecasted Value (2030) |
USD 23.3 billion |
|
CAGR (2024 – 2030) |
10.8% |
|
Base Year for Estimation |
2023 |
|
Historic Year |
2022 |
|
Forecast Period |
2024 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
eClinical Solutions Market By Solution (Clinical Trial Management Systems, Electronic Data Capture, Randomization and Trial Supply Management, Clinical Analytics Solutions, Regulatory Compliance Solutions, Patient Engagement Solutions), By Deployment Model (Cloud-Based Model, On-Premise Model), By Clinical Trial Phase (Phase I, Phase II, Phase III, Phase IV), and By End User (Pharmaceutical & Biopharmaceutical Companies, Contract Research Organizations, Hospitals & Healthcare Providers) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3.Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. eClinical Solutions Market, by Solution (Market Size & Forecast: USD Million, 2022 – 2030) |
|
4.1. Clinical Trial Management Systems |
|
4.2. Electronic Data Capture |
|
4.3. Randomization and Trial Supply Management |
|
4.4. Clinical Analytics Solutions |
|
4.5. Regulatory Compliance Solutions |
|
4.6. Patient Engagement Solutions |
|
4.7. Others |
|
5. eClinical Solutions Market, by Deployment Model (Market Size & Forecast: USD Million, 2022 – 2030) |
|
5.1. Cloud-Based Model |
|
5.2. On-Premise Model |
|
6. eClinical Solutions Market, by Clinical Trial Phase (Market Size & Forecast: USD Million, 2022 – 2030) |
|
6.1. Phase I |
|
6.2. Phase II |
|
6.3. Phase III |
|
6.4. Phase IV |
|
7. eClinical Solutions Market, by End User (Market Size & Forecast: USD Million, 2022 – 2030) |
|
7.1. Pharmaceutical & Biopharmaceutical Companies |
|
7.2. Contract Research Organizations |
|
7.3. Hospitals & Healthcare Providers |
|
7.4. Others |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2022 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America eClinical Solutions Market, by Solution |
|
8.2.7. North America eClinical Solutions Market, by Deployment Model |
|
8.2.8. North America eClinical Solutions Market, by Clinical Trial Phase |
|
8.2.9. North America eClinical Solutions Market, by End User |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US eClinical Solutions Market, by Solution |
|
8.2.10.1.2. US eClinical Solutions Market, by Deployment Model |
|
8.2.10.1.3. US eClinical Solutions Market, by Clinical Trial Phase |
|
8.2.10.1.4. US eClinical Solutions Market, by End User |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Clario |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Clinion |
|
10.3. eClinical Solutions |
|
10.4. ICON |
|
10.5. IQVIA |
|
10.6. Medidata |
|
10.7. Oracle |
|
10.8. Parexel International Corporation and |
|
10.9. Signant Health |
|
10.10. Veeva Systems Inc. |
|
11. Appendix |
Let us connect with you TOC
A comprehensive market research approach was employed to gather and analyze data on the eClinical Solutions Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the eClinical Solutions Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the eClinical Solutions ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the eClinical Solutions Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
Available Formats


