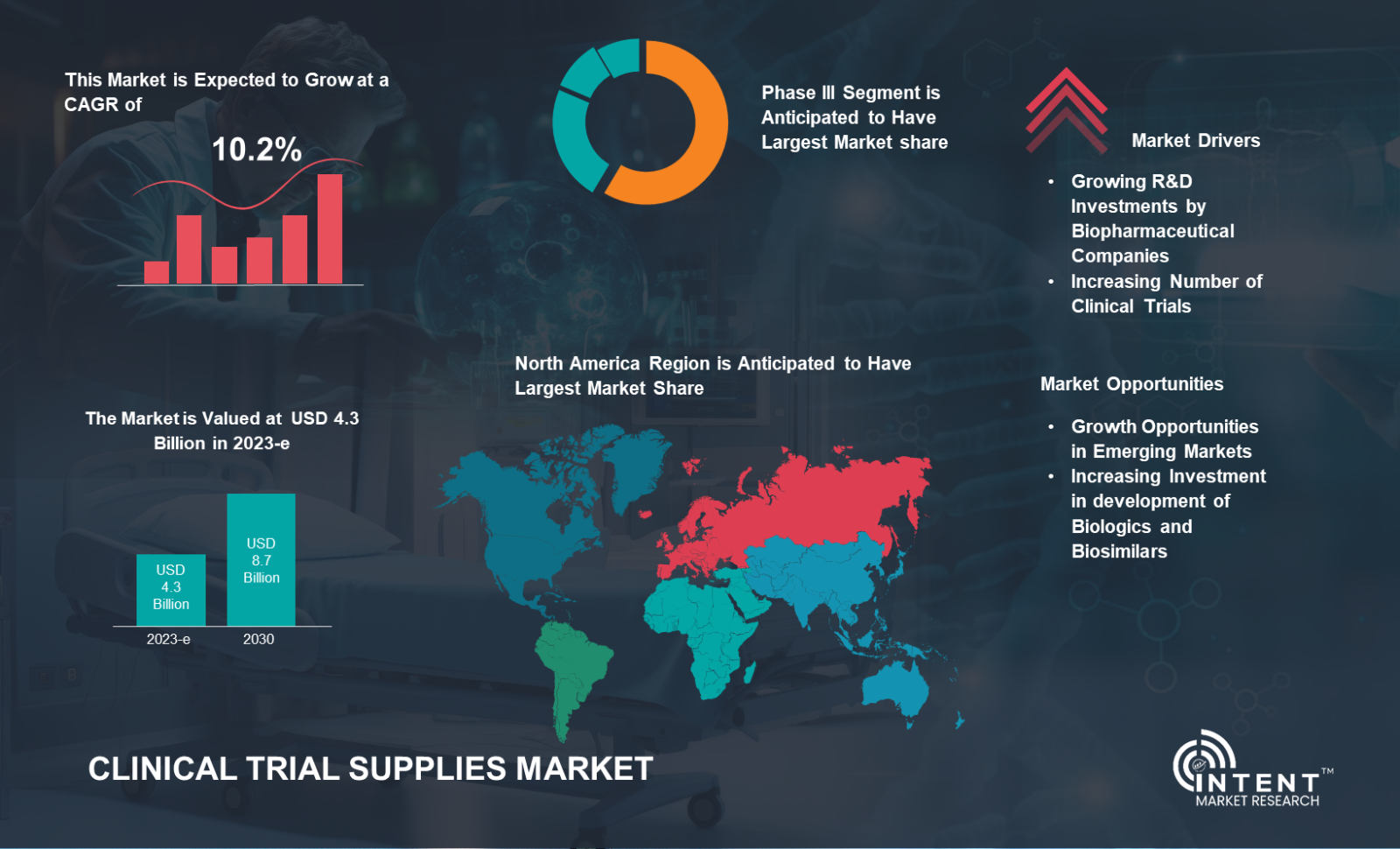
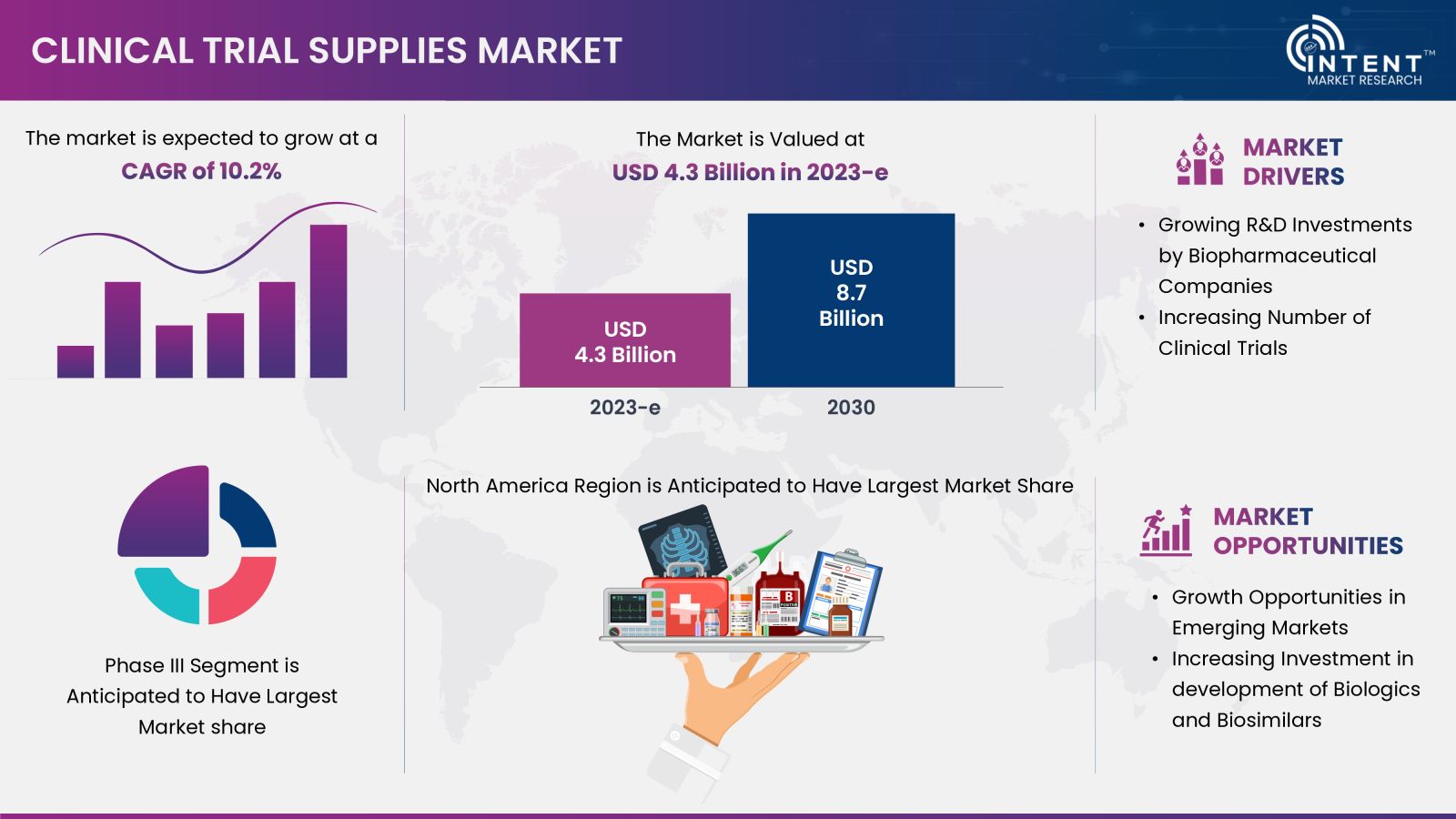
The Clinical Trial Supplies Market is expected to grow from USD 4.3 billion in 2023-e to USD 8.7 billion by 2030, at a CAGR of 10.2% during the forecast period. The clinical trial supplies market is a competitive market, the prominent players in the global market include Almac Group, Biocair, Catalent, Eurofins Scientific, Nuvisan, Lonza Group, PCI Pharma Services, Piramal Pharma Solutions, PRA Health Sciences, Sharp Services, and Thermo Fisher Scientific.
Clinical trials supplies are critical to the discovery of novel medications, medical equipment, treatments, and their proper execution depends on the availability of clinical trial materials. These supplies include medications under investigation, placebos, medical equipment, and other materials required for the research. The growth of the market is expected to be driven by factors such as partnerships and collaborations among market players for clinical trial outsourcing services, rising R&D spending by pharmaceutical and biopharmaceutical companies, and an increase in the number of clinical trials being conducted worldwide.
Clinical Trials Supplies Market is driven by Increasing R&D Spending by Biopharmaceutical Companies
The clinical trial supplies market is experiencing significant growth due to the escalating R&D investments by biopharmaceutical companies. This surge is fueled by the expanding scope of clinical trials undertaken by these companies, reflecting an intensified focus on innovative drug development and therapeutic interventions. The increasing number of clinical trials underscores the rising demand for various trial supplies, including investigational drugs, placebos, and medical devices. Moreover, the market is propelled by the imperative to meet strict regulatory requirements, ensuring the safety and efficacy of medical interventions.
Logistics & Distribution Services Segment, fueled by Leading Players Ensuring Effective Material Distribution
In 2023, logistics & distribution services segment accounted for the largest share of the clinical trial supplies market. The logistics and distribution segment is expected to witness significant growth over the forecast period due to the existence of notable players providing clinical trial services. These services include the effective administration of clinical trial material distribution and transportation, and guaranteeing prompt and safe delivery to many trial locations. This entails managing various logistical difficulties and putting into practice efficient supply chain plans.
Oncology Segment Dominates Clinical Trial Supplies Market Driven by Innovative Cancer Treatments
The oncology segment led the clinical trial supplies market in 2023. A significant portion of this market is driven by the relentless pursuit of innovative cancer treatments, with clinical trials necessitating a diverse range of supplies such as experimental drugs and specialized diagnostics. Neurology, another significant area, explores interventions for disorders of the nervous system, driving the demand for tailored trial supplies such as neurological medications and diagnostic equipment.
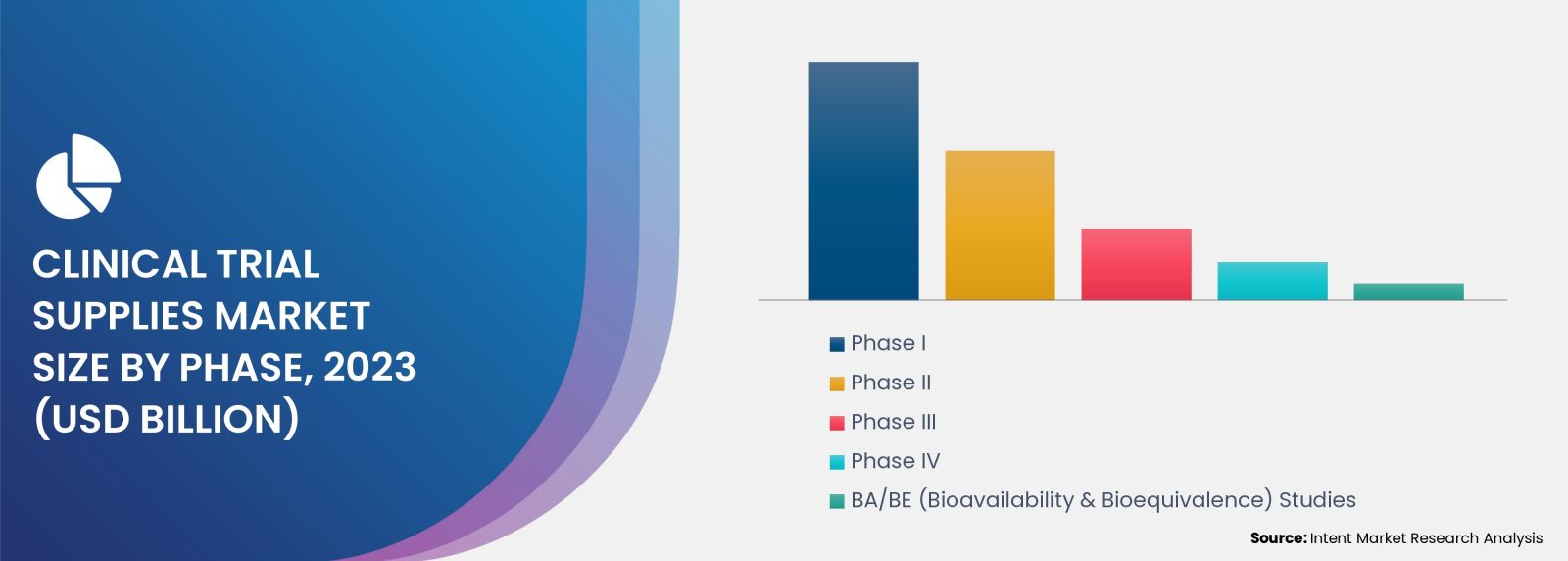
Phase III Emerges as the Pinnacle in Clinical Trial Supplies Market, Reflecting Industry's Focus and Evolution in Research
Phase III segment is the fastest-growing segment in the clinical trial supplies market. As drugs move closer to market approval, the demand for clinical trial supplies surges during these pivotal and resource-intensive trials. The complexities of Phase III trials, involving larger patient cohorts and global distribution, contribute to the accelerated growth of this market segment.
As the pharmaceutical industry continues to innovate and advance, the demand for Phase III clinical trial supplies is likely to remain robust, making it a key focus for stakeholders in the clinical trial supplies market. In 2020, 53.2% of this expenditure was allocated to phase III trials, 19.6% to phase II, and 15% to Phase I. By 2028 the expenditures for phase I and II trials is anticipated to further increase, reflecting the evolving landscape of clinical research and a growing focus on early and mid-stage trials in the coming years.
Pharmaceutical & Biopharmaceutical Dominates the Market with Increasing Clinical Trials
Pharmaceutical & biopharmaceutical companies segment held the largest share of the clinical trial supplies market in 2023. The increasing number of clinical trials conducted by pharmaceutical and biopharmaceutical firms propels the market. This uptick underscores the essential role that clinical trial supplies play in the rigorous testing of investigational drugs, placebos, and medical devices.
The increasing R&D Spending for Clinical Trials in the North America region is Driving the Clinical Trial Supplies Market
North America led the clinical trial supplies market in 2023 driven by an increase in R&D spending for clinical trials across region. The Pharmaceutical Research and Manufacturers of America (PhRMA) members spent 19% of their combined global revenues on R&D in 2022. The research and development (R&D) expenditure of its member companies in 2022 accounted for USD 101 billion worldwide.
Major Industry Players are Enhancing their Positions by Actively Developing Clinical Trials Platforms
The market is characterized by intense competition due to the presence of numerous international and domestic players. The clinical trial supplies market, in particular, is dominated by key players such as Almac Group, Biocair, Catalent, Eurofins Scientific, Nuvisan, Lonza Group, PCI Pharma Services, Piramal Pharma Solutions, PRA Health Sciences, Sharp Services, and Thermo Fisher Scientific, amongst others. These industry leaders primarily focus on acquiring smaller players and innovating their product lines to cater to changing consumer preferences and needs. The success of market players is heavily dependent on their ability to adapt to changing market trends and consumer preferences.
- In July 2023, Thermo Fisher Scientific formed a partnership with the national minority quality forum (NMQF) aims to enhance the participation of historically underserved patient groups in clinical research.
- In April 2023, Almac Group launced the IXRS3 Partnership Network, designed to streamline the development and implementation of advanced eClinical solutions for biopharmaceutical sponsors.
Clinical Trial Supplies Market Coverage
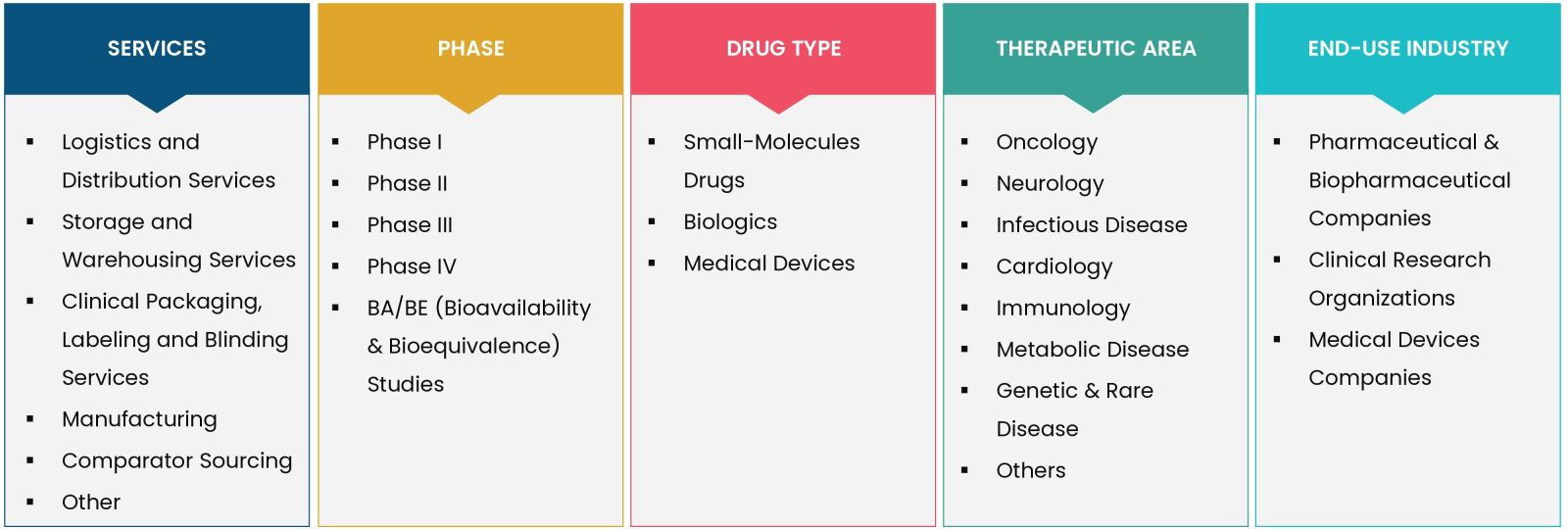
The report provides key insights into the clinical trial supplies market, and it focuses on technological developments, trends, and initiatives taken by the government in this sector. The report delves into market drivers, restraints, and opportunities, and analyses key players and the competitive landscape within the market
Report Scope
|
Report Features |
Description |
|
Market Size (2023-e) |
USD 4.3 billion |
|
Forecast Revenue (2030) |
USD 8.7 billion |
|
CAGR (2024-2030) |
10.2% |
|
Base Year for Estimation |
2023-e |
|
Historic Year |
2022 |
|
Forecast Period |
2024-2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
By Services (Logistics & Distribution Services, Storage & Warehousing Services, Clinical Packaging, Labeling & Blinding Services, Manufacturing, Comparator Sourcing, Others), By Phase (Phase I, Phase II, Phase III, Phase IV, BA/BE) Studies), By Drug Type (Small-Molecules Drugs, Biologics, Medical Devices), By Therapeutic Area (Oncology, Neurology, Infectious Disease, Cardiology, Immunology, Metabolic Disease, Genetic & Rare Disease, Others), By End-use (Pharmaceutical & Biopharmaceutical Companies, Clinical Research Organizations, Medical Devices Companies) |
|
Regional Analysis |
North America (US, Canada), Europe (Germany, France, UK, Spain, Italy), Asia-Pacific (China, Japan, South Korea, India), Latin America (Brazil, Mexico, Argentina), Middle East and Africa (Saudi Arabia, South Africa, Turkey, United Arab Emirates) |
|
Competitive Landscape |
Almac Group, Biocair, Catalent, Eurofins Scientific, Nuvisan, Lonza Group, PCI Pharma Services, Piramal Pharma Solutions, PRA Health Sciences, Sharp Services, and Thermo Fisher Scientific |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. |
|
Purchase Options |
We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) |
|
1.Introduction |
|
1.1.Study Assumptions and Market Definition |
|
1.2.Scope of the Study |
|
2.Research Methodology |
|
3.Executive Summary |
|
4.Market Dynamics |
|
4.1.Market Growth Drivers |
|
4.1.1.Growing R&D Spending by Biopharmaceutical Companies |
|
4.1.2.Increasing Number of Clinical Trials Conducted by Biopharmaceutical Companies |
|
4.1.3.Strict Regulatory Requirements to Ensure the Safety and Efficacy |
|
4.1.4.Increasing Outsourcing Activities |
|
4.1.5.Increasing Prevalence Of Diseases Such As Orphan And Rare Disease |
|
4.2.Market Growth Restraints |
|
4.2.1.High Cost of Drug Development |
|
4.3.Market Growth Opportunities |
|
4.3.1.Growth Opportunity in Emerging Markets |
|
4.3.2.Increasing Investments in the Development of Biologics and Biosimilar |
|
4.4.Pestle Analysis |
|
4.5.Porter’s Five Forces Analysis |
|
5.Market Outlook |
|
5.1.Overview (Industry Snapshot) |
|
5.2.Technology Analysis |
|
5.3.Supply Chain Analysis |
|
5.4.Value Chain Analysis |
|
5.5.Regulatory Analysis |
|
5.6.Reimbursement Analysis |
|
5.7.Pricing Analysis |
|
5.8.Patent Analysis |
|
5.9.Unmet needs in the market |
|
5.10. Trends/Disruptions Impacting Customer's Businesses |
|
5.11. Key Conference and Events |
|
6.Market Segment Outlook (Market Size & Forecast: USD Billion, 2023 – 2030) |
|
6.1.Segment Synopsis |
|
6.2.By Services |
|
6.2.1.Storage and Warehousing Services |
|
6.2.2.Logistics and Distribution Services |
|
6.2.3.Clinical Packaging, Labeling, and Blinding Services |
|
6.2.4.Comparator Sourcing |
|
6.2.5.Manufacturing |
|
6.2.6.Others |
|
6.3.By Phase |
|
6.3.1.Phase I |
|
6.3.2.Phase II |
|
6.3.3.Phase III |
|
6.3.4.Phase IV |
|
6.3.5.BA/BE (Bioavailability & Bioequivalence) Studies |
|
6.4.By Drug Type |
|
6.4.1.Small-Molecules Drugs |
|
6.4.2.Biologics |
|
6.4.3.Medical Devices |
|
6.5.By Therapeutic Area |
|
6.5.1.Oncology |
|
6.5.2.Neurology |
|
6.5.3.Infectious Disease |
|
6.5.4.Cardiology |
|
6.5.5.Immunology |
|
6.5.6.Metabolic Disease |
|
6.5.7.Genetic & Rare Disease |
|
6.5.8.Others |
|
6.6.By End-Use |
|
6.6.1.Pharmaceutical & Biopharmaceutical Companies |
|
6.6.2.Clinical Research Organizations |
|
6.6.3.Medical Devices Companies |
|
7.Regional Outlook (Market Size & Forecast: USD Billion, 2023 – 2030) |
|
7.1.Global Market Synopsis |
|
7.2.North America |
|
7.2.1.North America Clinical Trial Supplies Market Outlook |
|
7.2.1.1.US Clinical Trial Supplies Market, By Services |
|
7.2.1.2.US Clinical Trial Supplies Market, By Phase |
|
7.2.1.3.US Clinical Trial Supplies Market, By Drug Type |
|
7.2.1.4.US Clinical Trial Supplies Market, By Therapeutic Area |
|
7.2.1.5.US Clinical Trial Supplies Market, By End-Use Industry |
|
*Note: Cross-segmentation by segments for each country will be covered as shown above. |
|
7.2.2.Canada |
|
7.3.Europe |
|
7.3.1.Europe Clinical Trial Supplies Market Outlook |
|
7.3.2.Germany |
|
7.3.3.UK |
|
7.3.4.France |
|
7.3.5.Spain |
|
7.3.6.Italy |
|
7.4.Asia-Pacific |
|
7.4.1.Asia-Pacific Clinical Trial Supplies Market Outlook |
|
7.4.2.China |
|
7.4.3.India |
|
7.4.4.Japan |
|
7.4.5.South Korea |
|
7.4.6.Australia |
|
7.5.Latin America |
|
7.5.1.Latin America Clinical Trial Supplies Market Outlook |
|
7.5.2.Mexico |
|
7.5.3.Brazil |
|
7.6.Middle East & Africa |
|
7.6.1.Middle East & Africa Clinical Trial Supplies Market Outlook |
|
7.6.2.Saudi Arabia |
|
7.6.3.UAE |
|
8.Competitive Landscape |
|
8.1.Market Share Analysis |
|
8.2.Volume Output Analysis |
|
8.3.Company Strategy Analysis |
|
8.4.Competitive Matrix |
|
9.Company Profiles |
|
9.1.Almac Group |
|
9.1.1.Company Synopsis |
|
9.1.2.Company Financials |
|
9.1.3.Product/Service Portfolio |
|
9.1.4.Recent Developments |
|
9.1.5.Analyst Perception |
|
*Note: All the companies in section 9.1 will cover the same sub-chapters as above. |
|
9.2.Biocair |
|
9.3.Catalent |
|
9.4.Eurofins Scientific |
|
9.5.Nuvisan |
|
9.6.Lonza Group |
|
9.7.PCI Pharma Services |
|
9.8.Piramal Pharma Solutions |
|
9.9.PRA Health Sciences |
|
9.10.Sharp Services |
|
9.11.Thermo Fisher Scientific |
Intent Market Research employs a rigorous methodology to minimize residual errors by carefully defining the scope, validating findings through primary research, and consistently updating our in-house database. This dynamic approach allows us to capture ongoing market fluctuations and adapt to evolving market uncertainties.
The research factors used in our methodology vary depending on the specific market being analyzed. To begin with, we incorporate both demand and supply side information into our model to identify and address market gaps. Additionally, we also employ approaches such as Macro-Indicator Analysis, Factor Analysis, Value Chain-Based Sizing, and forecasting to further increase the accuracy of the numbers and validate the findings.
Research Approach
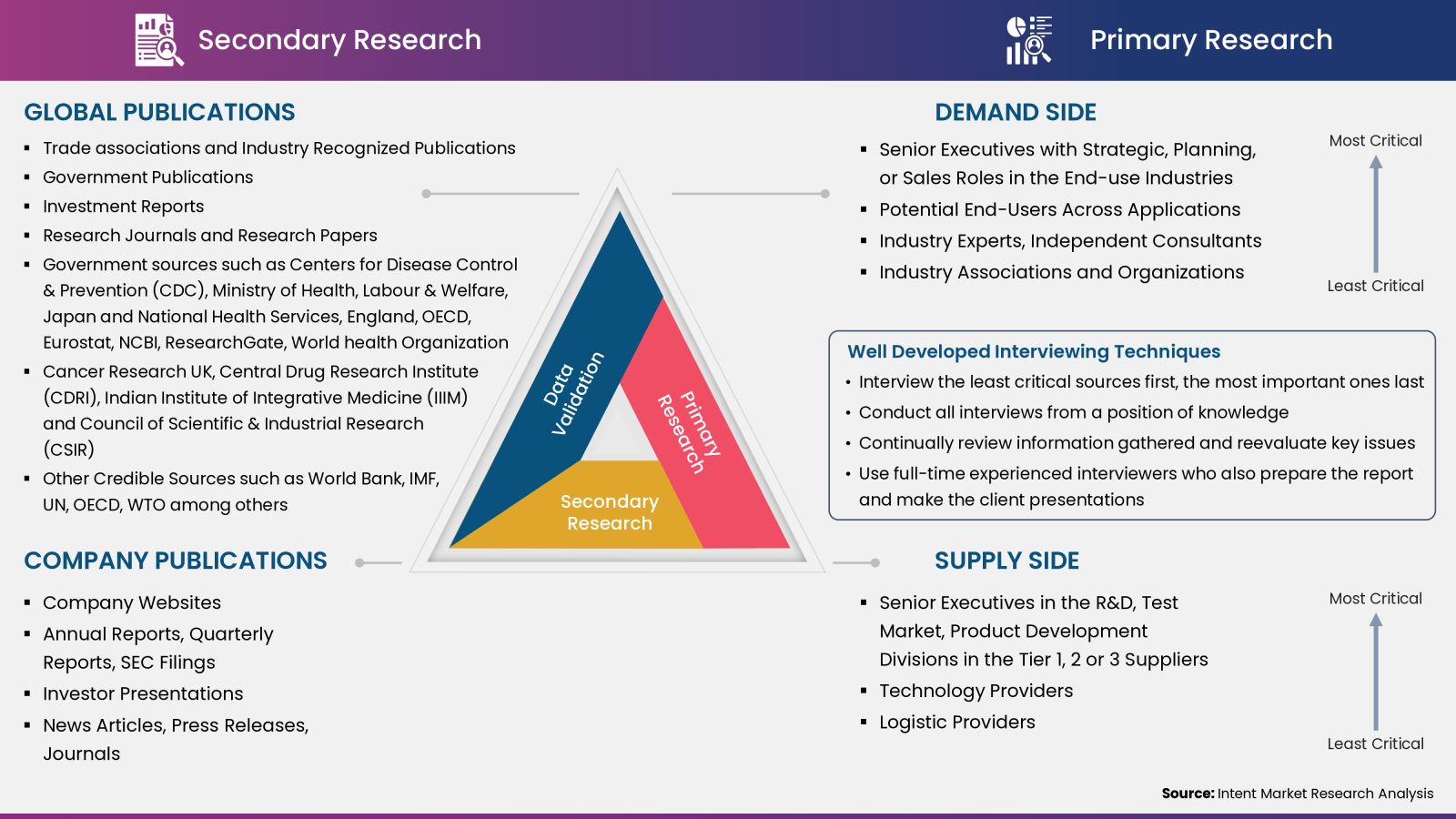
- Secondary Research Approach: During the initial phase of the research process, we acquire and accumulate extensive data continuously. This data is carefully filtered and validated through a variety of secondary sources.
- Primary Research Approach: Following the consolidation of data gathered through secondary research, we initiate a validation and verification process to verify all the market numbers and assumptions by engaging with the subject matter experts.
Data Collection, Analysis and Interpretation:
Research Methodology
Our market research methodology utilizes both top-down and bottom-up approaches to segment and estimate quantitative aspects of the market. We also employ multi-perspective analysis, examining the market from distinct viewpoints.