As per Intent Market Research, the Angioimmunoblastic T-Cell Lymphoma Market was valued at USD 2.2 billion in 2023 and will surpass USD 4.6 billion by 2030; growing at a CAGR of 10.9% during 2024 - 2030.
The Angioimmunoblastic T-Cell Lymphoma (AITL) Treatment Market is expanding rapidly due to the increasing prevalence of this rare and aggressive form of non-Hodgkin lymphoma. AITL primarily affects older adults and is characterized by the uncontrolled growth of T-cells, which are a part of the immune system. While the treatment landscape for AITL has traditionally been limited, recent advancements in chemotherapy, immunotherapy, and targeted therapies have provided significant opportunities to improve patient outcomes. The market is segmented by treatment type, drug class, route of administration, and end-user, each contributing to the dynamic landscape of AITL treatment. Below, we delve into the key sub-segments driving growth and innovation in this space.
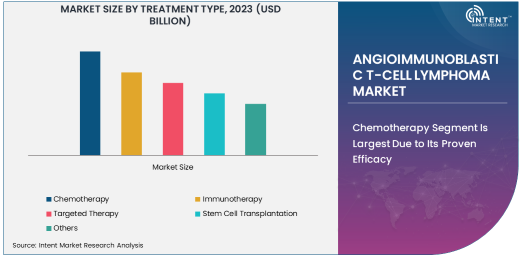
Chemotherapy Segment Is Largest Due to Its Proven Efficacy
Chemotherapy remains the largest sub-segment in the treatment type segment for angioimmunoblastic T-cell lymphoma (AITL). Chemotherapy, often as a combination therapy, has long been the mainstay treatment for AITL and other forms of lymphoma. The use of chemotherapy agents like cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP regimen) is common in AITL, aiming to rapidly reduce the tumor burden and manage symptoms. This treatment modality is widely available, well-established, and can be used to address the aggressive nature of AITL, especially in cases where the disease has already spread.
The relatively high efficacy of chemotherapy in inducing remission, especially in the initial stages of treatment, has sustained its dominance in the market. While new treatment options like immunotherapy and targeted therapy are gaining ground, chemotherapy remains a crucial option, particularly for patients who cannot access newer therapies or in cases where rapid tumor shrinkage is required. As the standard of care for many AITL patients, chemotherapy will continue to lead the treatment market, though it may be increasingly used in combination with emerging therapies.
Monoclonal Antibodies Are Largest Drug Class Due to Their Effectiveness in Targeting Cancer Cells
In the drug class segment, monoclonal antibodies represent the largest sub-segment. Monoclonal antibodies are lab-created molecules designed to target specific antigens on cancer cells, offering a highly targeted treatment option for AITL. Rituximab, one of the most commonly used monoclonal antibodies, has shown efficacy in treating B-cell lymphomas and, in combination with chemotherapy, has demonstrated some effectiveness in T-cell lymphomas like AITL. Additionally, newer monoclonal antibodies that target different cancer antigens are emerging as promising options for AITL treatment.
The popularity of monoclonal antibodies stems from their ability to specifically target cancer cells while sparing normal, healthy cells, which leads to a reduced risk of side effects compared to conventional chemotherapy. As the use of monoclonal antibodies in oncology expands, their adoption in the treatment of AITL will continue to grow, solidifying their position as the largest drug class in this market.
Intravenous Route of Administration Is Largest Due to Direct Delivery and Faster Action
In the route of administration segment, intravenous (IV) delivery remains the largest sub-segment for angioimmunoblastic T-cell lymphoma treatments. IV administration is commonly used for chemotherapy agents, monoclonal antibodies, and immunotherapy, as it allows for direct and rapid delivery of the drug into the bloodstream, providing faster therapeutic effects. For conditions like AITL, where prompt and aggressive treatment is essential, intravenous delivery offers a significant advantage in terms of bioavailability and speed of action.
Many of the most effective treatments for AITL, such as chemotherapy and monoclonal antibodies, are administered intravenously, making this route the preferred choice for both clinicians and patients. Moreover, intravenous administration is often necessary for the administration of biologics and immunotherapies, which are typically not effective when given orally. As a result, intravenous administration will continue to dominate the AITL treatment market, particularly for those requiring systemic therapies.
Hospitals End-User Segment Is Largest Due to Acute and Specialized Care
In the end-user segment, hospitals represent the largest sub-segment in the angioimmunoblastic T-cell lymphoma treatment market. Hospitals are the primary settings for the treatment of AITL, especially in advanced stages where patients require intensive chemotherapy, immunotherapy, or stem cell transplantation. The presence of specialized oncology units, skilled medical professionals, and the infrastructure to provide supportive care, such as blood transfusions and infection management, makes hospitals the ideal place for AITL treatment.
AITL is a rare and aggressive lymphoma that often requires a combination of therapies and close monitoring, which is best provided in a hospital setting. Hospitals are also better equipped to manage the potential side effects of treatment, including neutropenia, infection, and organ toxicity, which are common with aggressive therapies like chemotherapy and stem cell transplantation. As the need for specialized care grows, hospitals will continue to be the largest end-user segment for AITL treatments.
North America Is Largest Region Due to Advanced Medical Infrastructure
North America is the largest regional market for angioimmunoblastic T-cell lymphoma (AITL) treatments, primarily driven by the region's advanced healthcare infrastructure, high levels of healthcare expenditure, and robust clinical research environment. The United States, in particular, is a major contributor to the market, with its well-established oncology centers, high patient awareness, and access to the latest cancer therapies. North American countries also have strong regulatory frameworks that enable quicker approval of innovative therapies, including immunotherapies and targeted treatments for lymphoma.
The increasing prevalence of non-Hodgkin lymphoma in the region, combined with the availability of cutting-edge treatments such as monoclonal antibodies and immune checkpoint inhibitors, is expected to drive market growth. Furthermore, the high rate of participation in clinical trials and the strong focus on precision medicine in North America are contributing factors to the rapid development and adoption of new treatments for AITL. As a result, North America will remain the largest market for AITL treatments in the coming years.

Leading Companies & Competitive Landscape
The Angioimmunoblastic T-Cell Lymphoma (AITL) treatment market is highly competitive, with numerous pharmaceutical and biotechnology companies competing to develop innovative therapies. Leading players in the market include pharmaceutical giants like Roche, Merck, Bristol-Myers Squibb, and Novartis, which are actively involved in developing and commercializing immunotherapies, monoclonal antibodies, and targeted treatments for AITL and other types of lymphoma. Additionally, companies like Gilead Sciences and AbbVie are contributing to the market with their development of targeted therapies and new drug combinations.
The market is witnessing an influx of new players focusing on immuno-oncology, with several promising immune checkpoint inhibitors and monoclonal antibodies entering clinical trials for AITL. With the rapid pace of innovation, collaborations between pharmaceutical companies, research institutions, and oncology centers are becoming increasingly common. As competition intensifies, companies are focusing on expanding their portfolios and enhancing their research capabilities to meet the growing demand for more effective and less toxic treatments for AITL. The evolving treatment landscape will continue to foster innovation, benefiting patients with this aggressive lymphoma.
Recent Developments:
- Bristol Myers Squibb announced the successful phase III trial results of its immunotherapy for AITL, showing promising efficacy.
- Novartis received FDA approval for a new targeted therapy specifically for T-cell lymphomas, including AITL.
- Merck & Co., Inc. entered into a collaboration agreement with a biotech firm to develop a new monoclonal antibody treatment for AITL.
- AbbVie Inc. launched a combination therapy that showed increased survival rates in patients with Angioimmunoblastic T-Cell Lymphoma.
- Eli Lilly and Company completed a clinical trial for a new tyrosine kinase inhibitor that could be effective for treating AITL.
List of Leading Companies:
- Bristol Myers Squibb
- Novartis International AG
- Merck & Co., Inc.
- Roche Holding AG
- AbbVie Inc.
- Amgen Inc.
- Gilead Sciences
- Eli Lilly and Company
- Johnson & Johnson
- Pfizer Inc.
- Takeda Pharmaceuticals
- Celgene Corporation (now part of Bristol Myers Squibb)
- AstraZeneca PLC
- Sanofi S.A.
- Regeneron Pharmaceuticals
Report Scope:
|
Report Features |
Description |
|
Market Size (2023) |
USD 2.2 Billion |
|
Forecasted Value (2030) |
USD 4.6 Billion |
|
CAGR (2024 – 2030) |
10.9% |
|
Base Year for Estimation |
2023 |
|
Historic Year |
2022 |
|
Forecast Period |
2024 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
Angioimmunoblastic T-Cell Lymphoma Market By Treatment Type (Chemotherapy, Immunotherapy, Targeted Therapy, Stem Cell Transplantation), By Drug Class (Chemotherapy Agents, Monoclonal Antibodies, Immune Checkpoint Inhibitors, Tyrosine Kinase Inhibitors, Corticosteroids), By Route of Administration (Oral, Intravenous, Subcutaneous), By End-User (Hospitals, Cancer Research Institutes, Ambulatory Surgical Centers) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Major Companies |
Bristol Myers Squibb, Novartis International AG, Merck & Co., Inc., Roche Holding AG, AbbVie Inc., Amgen Inc., Eli Lilly and Company, Johnson & Johnson, Pfizer Inc., Takeda Pharmaceuticals, Celgene Corporation (now part of Bristol Myers Squibb), AstraZeneca PLC, Regeneron Pharmaceuticals |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3. Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. Angioimmunoblastic T-Cell Lymphoma Market, by Treatment Type (Market Size & Forecast: USD Million, 2022 – 2030) |
|
4.1. Chemotherapy |
|
4.2. Immunotherapy |
|
4.3. Targeted Therapy |
|
4.4. Stem Cell Transplantation |
|
4.5. Others |
|
5. Angioimmunoblastic T-Cell Lymphoma Market, by Drug Class (Market Size & Forecast: USD Million, 2022 – 2030) |
|
5.1. Chemotherapy Agents |
|
5.2. Monoclonal Antibodies |
|
5.3. Immune Checkpoint Inhibitors |
|
5.4. Tyrosine Kinase Inhibitors |
|
5.5. Corticosteroids |
|
5.6. Others |
|
6. Angioimmunoblastic T-Cell Lymphoma Market, by Route of Administration (Market Size & Forecast: USD Million, 2022 – 2030) |
|
6.1. Oral |
|
6.2. Intravenous |
|
6.3. Subcutaneous |
|
7. Angioimmunoblastic T-Cell Lymphoma Market, by End-User (Market Size & Forecast: USD Million, 2022 – 2030) |
|
7.1. Hospitals |
|
7.2. Cancer Research Institutes |
|
7.3. Ambulatory Surgical Centers |
|
7.4. Others |
|
8. Regional Analysis (Market Size & Forecast: USD Million, 2022 – 2030) |
|
8.1. Regional Overview |
|
8.2. North America |
|
8.2.1. Regional Trends & Growth Drivers |
|
8.2.2. Barriers & Challenges |
|
8.2.3. Opportunities |
|
8.2.4. Factor Impact Analysis |
|
8.2.5. Technology Trends |
|
8.2.6. North America Angioimmunoblastic T-Cell Lymphoma Market, by Treatment Type |
|
8.2.7. North America Angioimmunoblastic T-Cell Lymphoma Market, by Drug Class |
|
8.2.8. North America Angioimmunoblastic T-Cell Lymphoma Market, by Route of Administration |
|
8.2.9. North America Angioimmunoblastic T-Cell Lymphoma Market, by End-User |
|
8.2.10. By Country |
|
8.2.10.1. US |
|
8.2.10.1.1. US Angioimmunoblastic T-Cell Lymphoma Market, by Treatment Type |
|
8.2.10.1.2. US Angioimmunoblastic T-Cell Lymphoma Market, by Drug Class |
|
8.2.10.1.3. US Angioimmunoblastic T-Cell Lymphoma Market, by Route of Administration |
|
8.2.10.1.4. US Angioimmunoblastic T-Cell Lymphoma Market, by End-User |
|
8.2.10.2. Canada |
|
8.2.10.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
8.3. Europe |
|
8.4. Asia-Pacific |
|
8.5. Latin America |
|
8.6. Middle East & Africa |
|
9. Competitive Landscape |
|
9.1. Overview of the Key Players |
|
9.2. Competitive Ecosystem |
|
9.2.1. Level of Fragmentation |
|
9.2.2. Market Consolidation |
|
9.2.3. Product Innovation |
|
9.3. Company Share Analysis |
|
9.4. Company Benchmarking Matrix |
|
9.4.1. Strategic Overview |
|
9.4.2. Product Innovations |
|
9.5. Start-up Ecosystem |
|
9.6. Strategic Competitive Insights/ Customer Imperatives |
|
9.7. ESG Matrix/ Sustainability Matrix |
|
9.8. Manufacturing Network |
|
9.8.1. Locations |
|
9.8.2. Supply Chain and Logistics |
|
9.8.3. Product Flexibility/Customization |
|
9.8.4. Digital Transformation and Connectivity |
|
9.8.5. Environmental and Regulatory Compliance |
|
9.9. Technology Readiness Level Matrix |
|
9.10. Technology Maturity Curve |
|
9.11. Buying Criteria |
|
10. Company Profiles |
|
10.1. Bristol Myers Squibb |
|
10.1.1. Company Overview |
|
10.1.2. Company Financials |
|
10.1.3. Product/Service Portfolio |
|
10.1.4. Recent Developments |
|
10.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
10.2. Novartis International AG |
|
10.3. Merck & Co., Inc. |
|
10.4. Roche Holding AG |
|
10.5. AbbVie Inc. |
|
10.6. Amgen Inc. |
|
10.7. Gilead Sciences |
|
10.8. Eli Lilly and Company |
|
10.9. Johnson & Johnson |
|
10.10. Pfizer Inc. |
|
10.11. Takeda Pharmaceuticals |
|
10.12. Celgene Corporation (now part of Bristol Myers Squibb) |
|
10.13. AstraZeneca PLC |
|
10.14. Sanofi S.A. |
|
10.15. Regeneron Pharmaceuticals |
|
11. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the Angioimmunoblastic T-Cell Lymphoma Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the Angioimmunoblastic T-Cell Lymphoma Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the E-Waste Management ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the Angioimmunoblastic T-Cell Lymphoma Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
