As per Intent Market Research, the AI in Clinical Trials Market was valued at USD 1.8 billion in 2023 and will surpass USD 7.6 billion by 2030; growing at a CAGR of 23.0% during 2024 - 2030.
The AI in Clinical Trials Market is poised for significant growth, driven by advancements in technology and the increasing demand for efficient and effective clinical research processes. As the healthcare sector seeks to enhance patient outcomes and streamline drug development, artificial intelligence (AI) is becoming a crucial component in optimizing clinical trials. This market encompasses various applications of AI, including patient recruitment, data management, and predictive analytics, among others.

Patient Recruitment Segment is Largest Owing to Increased Efficiency
Among the various segments of the AI in Clinical Trials Market, patient recruitment is the largest, accounting for a significant share of the market revenue. The integration of AI in patient recruitment helps streamline the selection process by utilizing algorithms to analyze vast datasets, including electronic health records, genetic information, and demographic data. This targeted approach ensures that clinical trials can enroll the right participants more efficiently, significantly reducing the time and costs associated with traditional recruitment methods.
The need for faster patient recruitment has become increasingly critical, especially in light of the pressing timelines imposed by regulatory agencies and the growing demand for timely therapies. AI-powered platforms can enhance the recruitment process by identifying eligible candidates swiftly, thereby increasing the overall trial success rate. This effectiveness is expected to propel further investments into AI-driven patient recruitment solutions, making it a cornerstone of the AI in Clinical Trials Market.
Data Management Segment is Fastest Growing Owing to Rising Data Complexity
The data management segment within the AI in Clinical Trials Market is recognized as the fastest-growing segment, primarily due to the increasing complexity of clinical trial data. As clinical trials generate vast amounts of data from diverse sources, including genomic data, imaging studies, and patient-reported outcomes, the need for sophisticated data management solutions has never been greater. AI technologies such as machine learning and natural language processing are being deployed to analyze and manage this data efficiently, transforming raw data into actionable insights.
Moreover, regulatory compliance requirements are becoming more stringent, further necessitating robust data management solutions. AI-driven tools help ensure data integrity and facilitate real-time monitoring, which is crucial for compliance with regulatory standards. This trend is expected to drive the demand for advanced data management solutions, making it a vital area for growth within the AI in Clinical Trials Market.
Predictive Analytics Segment is Largest Owing to Enhanced Decision-Making
Predictive analytics is one of the largest subsegments within the AI in Clinical Trials Market, primarily due to its capability to enhance decision-making processes. By employing machine learning algorithms, predictive analytics can identify patterns and trends within historical trial data, enabling stakeholders to make informed predictions about trial outcomes and patient responses. This level of insight is invaluable in designing clinical trials that are not only more efficient but also more likely to succeed.
The use of predictive analytics in clinical trials allows researchers to optimize trial designs, select appropriate endpoints, and anticipate potential challenges before they arise. As a result, pharmaceutical companies and research organizations are increasingly adopting predictive analytics solutions to mitigate risks associated with clinical trials. This trend is expected to continue, positioning predictive analytics as a key driver of innovation in the AI in Clinical Trials Market.
Regulatory Compliance Segment is Fastest Growing Owing to Stringent Guidelines
The regulatory compliance segment within the AI in Clinical Trials Market is witnessing rapid growth due to the rising need for adherence to stringent regulatory guidelines. As regulatory bodies emphasize data transparency and integrity, the demand for AI-driven compliance solutions has surged. These solutions leverage AI technologies to streamline the process of ensuring that clinical trials meet all regulatory requirements, from initial submissions to ongoing monitoring.
AI systems can automate documentation processes, track compliance metrics, and generate real-time reports for regulatory agencies. This not only enhances the efficiency of compliance efforts but also reduces the likelihood of human errors that can lead to costly delays or penalties. Consequently, the regulatory compliance segment is emerging as one of the fastest-growing areas within the AI in Clinical Trials Market, reflecting the critical importance of meeting regulatory standards in modern clinical research.
Site Selection Segment is Largest Owing to Increased Site Efficiency
The site selection segment is recognized as the largest in the AI in Clinical Trials Market, driven by the need for efficient trial site management. Effective site selection is crucial for the success of clinical trials, as it can significantly impact patient recruitment, data quality, and overall trial timelines. AI technologies are increasingly being utilized to analyze site performance data, historical patient populations, and logistical considerations to identify the most suitable trial sites.
By leveraging AI for site selection, sponsors can ensure that trials are conducted in locations with the best potential for patient enrollment and engagement. This targeted approach not only enhances site efficiency but also optimizes resource allocation, making it a key focus area for stakeholders in the clinical research landscape. The growing emphasis on site performance optimization positions the site selection segment as a pivotal player in the AI in Clinical Trials Market.
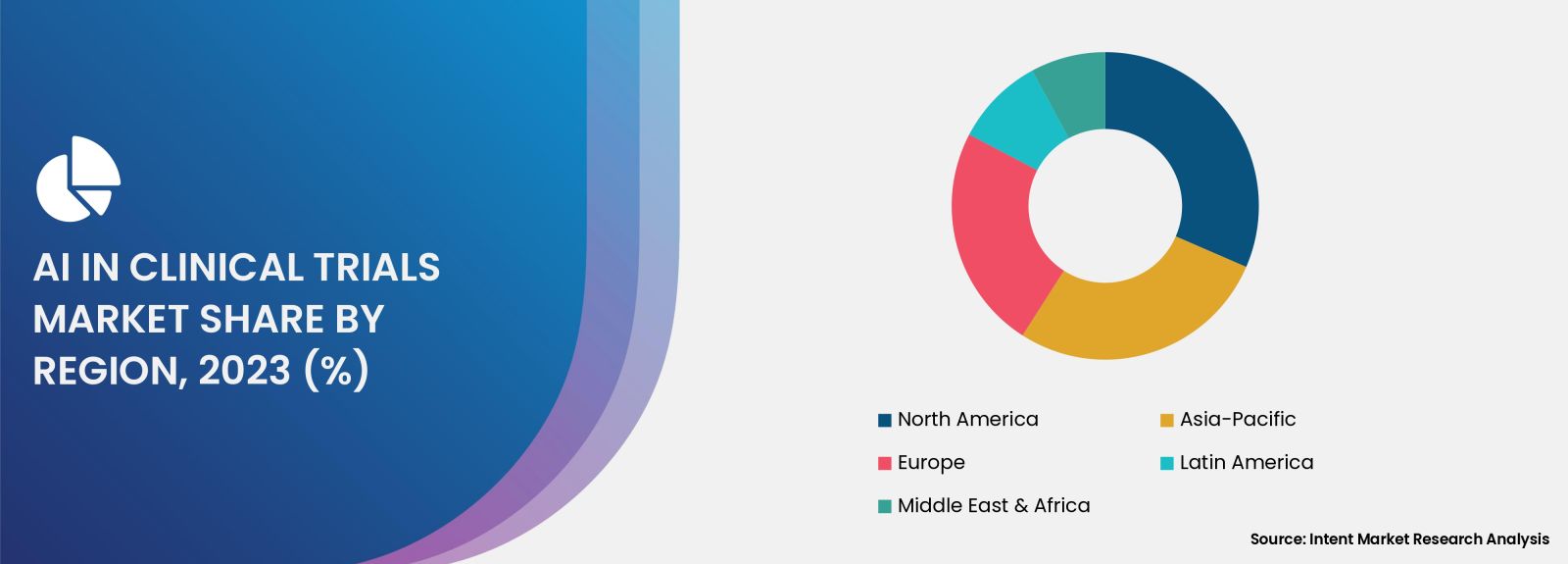
North America is the Largest Region Owing to Advanced Healthcare Infrastructure
North America is currently the largest region in the AI in Clinical Trials Market, driven by its advanced healthcare infrastructure, strong research capabilities, and a high level of investment in technological innovations. The region is home to numerous pharmaceutical companies, research institutions, and biotechnology firms that are actively leveraging AI to enhance clinical trial processes. With a robust ecosystem that supports research and development, North America is leading the way in adopting AI solutions for clinical trials.
Additionally, regulatory agencies in the region, such as the U.S. Food and Drug Administration (FDA), are increasingly recognizing the potential of AI in improving clinical trial efficiency and patient safety. This favorable regulatory environment, coupled with the presence of key industry players and abundant funding opportunities, positions North America as a dominant force in the AI in Clinical Trials Market. The region's commitment to innovation and technological advancement is expected to drive continued growth and development in this sector.
Competitive Landscape and Leading Companies
The competitive landscape of the AI in Clinical Trials Market is characterized by the presence of several key players that are at the forefront of technological innovation. Leading companies such as IBM Watson Health, Medidata Solutions, Oracle Corporation, and Siemens Healthineers are actively developing AI-driven solutions tailored to meet the needs of clinical trial stakeholders. These companies are focusing on strategic partnerships, mergers, and acquisitions to enhance their product offerings and expand their market reach.
The market is witnessing a surge in collaborations between technology firms and pharmaceutical companies, aimed at developing integrated solutions that enhance clinical trial efficiency. Additionally, new entrants are emerging with innovative AI applications designed to address specific challenges within the clinical trial process. As competition intensifies, companies are increasingly prioritizing research and development efforts to stay ahead in this dynamic and rapidly evolving market. The ongoing advancements in AI technologies are expected to further reshape the competitive landscape, paving the way for new business models and opportunities in the AI in Clinical Trials Market.
Report Objectives:
The report will help you answer some of the most critical questions in the AI in Clinical Trials Market. A few of them are as follows:
- What are the key drivers, restraints, opportunities, and challenges influencing the market growth?
- What are the prevailing technology trends in the AI in Clinical Trials Market?
- What is the size of the AI in Clinical Trials Market based on segments, sub-segments, and regions?
- What is the size of different market segments across key regions: North America, Europe, Asia-Pacific, Latin America, Middle East & Africa?
- What are the market opportunities for stakeholders after analyzing key market trends?
- Who are the leading market players and what are their market share and core competencies?
- What is the degree of competition in the market and what are the key growth strategies adopted by leading players?
- What is the competitive landscape of the market, including market share analysis, revenue analysis, and a ranking of key players?
Report Scope:
|
Report Features |
Description |
|
Market Size (2023) |
USD 1.8 billion |
|
Forecasted Value (2030) |
USD 7.6 billion |
|
CAGR (2024 – 2030) |
23.0% |
|
Base Year for Estimation |
2023 |
|
Historic Year |
2022 |
|
Forecast Period |
2024 – 2030 |
|
Report Coverage |
Market Forecast, Market Dynamics, Competitive Landscape, Recent Developments |
|
Segments Covered |
AI in Clinical Trials Market By Offering (Software, Services), By Phase (Phase I, Phase II, Phase III), By Technology (Machine Learning, Natural Language Processing), By Application (Patient Recruitment & Retention, Data Management & Analysis, Monitoring & Compliance), By Therapeutic Area (Oncology, Neurological Diseases & Conditions, Cardiovascular Diseases), By End User (Pharmaceutical & Biotechnology Companies, Contract Research Organizations) |
|
Regional Analysis |
North America (US, Canada, Mexico), Europe (Germany, France, UK, Italy, Spain, and Rest of Europe), Asia-Pacific (China, Japan, South Korea, Australia, India, and Rest of Asia-Pacific), Latin America (Brazil, Argentina, and Rest of Latin America), Middle East & Africa (Saudi Arabia, UAE, Rest of Middle East & Africa) |
|
Customization Scope |
Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements |
|
1. Introduction |
|
1.1. Market Definition |
|
1.2. Scope of the Study |
|
1.3. Research Assumptions |
|
1.4. Study Limitations |
|
2. Research Methodology |
|
2.1. Research Approach |
|
2.1.1. Top-Down Method |
|
2.1.2. Bottom-Up Method |
|
2.1.3. Factor Impact Analysis |
|
2.2. Insights & Data Collection Process |
|
2.2.1. Secondary Research |
|
2.2.2. Primary Research |
|
2.3. Data Mining Process |
|
2.3.1. Data Analysis |
|
2.3.2. Data Validation and Revalidation |
|
2.3.3. Data Triangulation |
|
3.Executive Summary |
|
3.1. Major Markets & Segments |
|
3.2. Highest Growing Regions and Respective Countries |
|
3.3. Impact of Growth Drivers & Inhibitors |
|
3.4. Regulatory Overview by Country |
|
4. AI in Clinical Trials Market, by Offering (Market Size & Forecast: USD Million, 2022 – 2030) |
|
4.1. Software |
|
4.2. Services |
|
5. AI in Clinical Trials Market, by Phase (Market Size & Forecast: USD Million, 2022 – 2030) |
|
5.1. Phase I |
|
5.2. Phase II |
|
5.3. Phase III |
|
6. AI in Clinical Trials Market, by Technology (Market Size & Forecast: USD Million, 2022 – 2030) |
|
6.1. Machine Learning |
|
6.1.1. Deep Learning |
|
6.1.2. Supervised Learning |
|
6.1.3. Others |
|
6.2. Natural Learning Processing |
|
6.3. Others |
|
7. AI in Clinical Trials Market, by Therapeutic Area (Market Size & Forecast: USD Million, 2022 – 2030) |
|
7.1. Oncology |
|
7.2. Neurological Disease & Condition |
|
7.3. Cardiovascular Diseases |
|
7.4. Metabolic Diseases |
|
7.5. Infectious Disease |
|
7.6. Immunology Disease |
|
7.7. Others |
|
8. AI in Clinical Trials Market, by Application (Market Size & Forecast: USD Million, 2022 – 2030) |
|
8.1. Patient Recruitment & Retention |
|
8.2. Data Management & Analysis |
|
8.3. Predictive Analytics |
|
8.4. Trial Design Optimization |
|
8.5. Monitoring & Compliance |
|
8.6. Others |
|
9. AI in Clinical Trials Market, by End User (Market Size & Forecast: USD Million, 2022 – 2030) |
|
9.1. Pharmaceutical & Biotechnology Companies |
|
9.2. Contract Research Organizations |
|
9.3. Others |
|
10. Regional Analysis (Market Size & Forecast: USD Million, 2022 – 2030) |
|
10.1. Regional Overview |
|
10.2. North America |
|
10.2.1. Regional Trends & Growth Drivers |
|
10.2.2. Barriers & Challenges |
|
10.2.3. Opportunities |
|
10.2.4. Factor Impact Analysis |
|
10.2.5. Technology Trends |
|
10.2.6. North America AI in Clinical Trials Market, by Offering |
|
10.2.7. North America AI in Clinical Trials Market, by Phase |
|
10.2.8. North America AI in Clinical Trials Market, by Technology |
|
10.2.9. North America AI in Clinical Trials Market, by Therapeutic Area |
|
10.2.10. North America AI in Clinical Trials Market, by Application |
|
10.2.11. North America AI in Clinical Trials Market, by End User |
|
10.2.12. By Country |
|
10.2.12.1. US |
|
10.2.12.1.1. US AI in Clinical Trials Market, by Offering |
|
10.2.12.1.2. US AI in Clinical Trials Market, by Phase |
|
10.2.12.1.3. US AI in Clinical Trials Market, by Technology |
|
10.2.12.1.4. US AI in Clinical Trials Market, by Therapeutic Area |
|
10.2.12.1.5. US AI in Clinical Trials Market, by Application |
|
10.2.12.1.6. US AI in Clinical Trials Market, by End User |
|
10.2.12.2. Canada |
|
10.2.12.3. Mexico |
|
*Similar segmentation will be provided for each region and country |
|
10.3. Europe |
|
10.4. Asia-Pacific |
|
10.5. Latin America |
|
10.6. Middle East & Africa |
|
11. Competitive Landscape |
|
11.1. Overview of the Key Players |
|
11.2. Competitive Ecosystem |
|
11.2.1. Level of Fragmentation |
|
11.2.2. Market Consolidation |
|
11.2.3. Product Innovation |
|
11.3. Company Share Analysis |
|
11.4. Company Benchmarking Matrix |
|
11.4.1. Strategic Overview |
|
11.4.2. Product Innovations |
|
11.5. Start-up Ecosystem |
|
11.6. Strategic Competitive Insights/ Customer Imperatives |
|
11.7. ESG Matrix/ Sustainability Matrix |
|
11.8. Manufacturing Network |
|
11.8.1. Locations |
|
11.8.2. Supply Chain and Logistics |
|
11.8.3. Product Flexibility/Customization |
|
11.8.4. Digital Transformation and Connectivity |
|
11.8.5. Environmental and Regulatory Compliance |
|
11.9. Technology Readiness Level Matrix |
|
11.10. Technology Maturity Curve |
|
11.11. Buying Criteria |
|
12. Company Profiles |
|
12.1. Ardigen |
|
12.1.1. Company Overview |
|
12.1.2. Company Financials |
|
12.1.3. Product/Service Portfolio |
|
12.1.4. Recent Developments |
|
12.1.5. IMR Analysis |
|
*Similar information will be provided for other companies |
|
12.2. BioSymetrics |
|
12.3. Euretos |
|
12.4. Exscientia |
|
12.5. IBM Corporation |
|
12.6. Insilico Medicine |
|
12.7. Intel |
|
12.8. Koneksa Health |
|
12.9. Saama |
|
12.10. Unlearn.AI, Inc. |
|
13. Appendix |
A comprehensive market research approach was employed to gather and analyze data on the AI in Clinical Trials Market. In the process, the analysis was also done to analyze the parent market and relevant adjacencies to measure the impact of them on the AI in Clinical Trials Market. The research methodology encompassed both secondary and primary research techniques, ensuring the accuracy and credibility of the findings.
.jpg)
Secondary Research
Secondary research involved a thorough review of pertinent industry reports, journals, articles, and publications. Additionally, annual reports, press releases, and investor presentations of industry players were scrutinized to gain insights into their market positioning and strategies.
Primary Research
Primary research involved conducting in-depth interviews with industry experts, stakeholders, and market participants across the AI in Clinical Trials ecosystem. The primary research objectives included:
- Validating findings and assumptions derived from secondary research
- Gathering qualitative and quantitative data on market trends, drivers, and challenges
- Understanding the demand-side dynamics, encompassing end-users, component manufacturers, facility providers, and service providers
- Assessing the supply-side landscape, including technological advancements and recent developments
Market Size Assessment
A combination of top-down and bottom-up approaches was utilized to analyze the overall size of the AI in Clinical Trials Market. These methods were also employed to assess the size of various subsegments within the market. The market size assessment methodology encompassed the following steps:
- Identification of key industry players and relevant revenues through extensive secondary research
- Determination of the industry's supply chain and market size, in terms of value, through primary and secondary research processes
- Calculation of percentage shares, splits, and breakdowns using secondary sources and verification through primary sources
.jpg)
Data Triangulation
To ensure the accuracy and reliability of the market size, data triangulation was implemented. This involved cross-referencing data from various sources, including demand and supply side factors, market trends, and expert opinions. Additionally, top-down and bottom-up approaches were employed to validate the market size assessment.
NA
